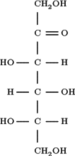
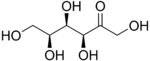
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.

A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
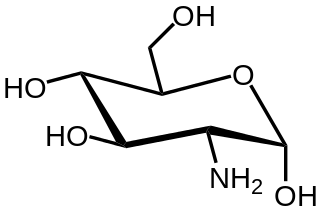
In organic chemistry, an amino sugar is a sugar molecule in which a hydroxyl group has been replaced with an amine group. More than 60 amino sugars are known, with one of the most abundant being N-Acetyl-d-glucosamine, which is the main component of chitin.
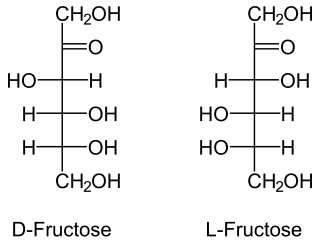
A ketose is a monosaccharide containing one ketone group per molecule. The simplest ketose is dihydroxyacetone, which has only three carbon atoms. It is the only ketose with no optical activity. All monosaccharide ketoses are reducing sugars, because they can tautomerize into aldoses via an enediol intermediate, and the resulting aldehyde group can be oxidised, for example in the Tollens' test or Benedict's test. Ketoses that are bound into glycosides, for example in the case of the fructose moiety of sucrose, are nonreducing sugars.

Benzyl alcohol (also known as α-Cresol) is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful as a solvent for its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure R−CH2−C6H5. Benzyl features a benzene ring attached to a methylene group group.

Tagatose is a hexose monosaccharide. It is found in small quantities in a variety of foods, and has attracted attention as an alternative sweetener. It is often found in dairy products, because it is formed when milk is heated. It is similar in texture and appearance to sucrose :215 and is 92% as sweet,:198 but with only 38% of the calories.:209 Tagatose is generally recognized as safe by the Food and Agriculture Organization and the World Health Organization, and has been since 2001. Since it is metabolized differently from sucrose, tagatose has a minimal effect on blood glucose and insulin levels. Tagatose is also approved as a tooth-friendly ingredient for dental products. Consumption of more than about 30 grams of tagatose in a dose may cause gastric disturbance in some people, as it is mostly processed in the large intestine, similar to soluble fiber.:214

Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl group. In its pure form it is a water-sensitive oily colorless liquid, although impure samples usually appear yellow. It possesses a characteristic pungent odor and degrades in contact with water.
Benzyl bromide is an organic compound with the formula C6H5CH2Br. The molecule consists of a benzene ring substituted with a bromomethyl group. It is a colorless liquid with lachrymatory properties. The compound is a reagent for introducing benzyl groups.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.

Prenalterol is a cardiac stimulant which acts as a β1 adrenoreceptor agonist.
In enzymology, a sorbose dehydrogenase is an enzyme that catalyzes the chemical reaction
In enzymology, a sorbose 5-dehydrogenase (NADP+) (EC 1.1.1.123) is an enzyme that catalyzes the chemical reaction
In enzymology, a sorbose reductase (EC 1.1.1.289) is an enzyme that catalyzes the chemical reaction
In enzymology, a L-sorbose oxidase (EC 1.1.3.11) is an enzyme that catalyzes the chemical reaction
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
The Reichstein process in chemistry is a combined chemical and microbial method for the production of ascorbic acid from D-glucose that takes place in several steps. This process was devised by Nobel Prize winner Tadeusz Reichstein and his colleagues in 1933 while working in the laboratory of the ETH in Zürich.
Fétizon oxidation is the oxidation of primary and secondary alcohols utilizing the compound silver(I) carbonate absorbed onto the surface of celite also known as Fétizon's reagent first employed by Marcel Fétizon in 1968. It is a mild reagent, suitable for both acid and base sensitive compounds. Its great reactivity with lactols makes the Fétizon oxidation a useful method to obtain lactones from a diol. The reaction is inhibited significantly by polar groups within the reaction system as well as steric hindrance of the α-hydrogen of the alcohol.

A rare sugar is a sugar that occurs in limited quantities in nature. Rare sugars can be made using enzymes, choosing which enzymes to use if you know the substrate can be aided by the Izumoring-strategy.
The PTS Mannose-Fructose-Sorbose (Man) Family is a group of multicomponent PTS systems that are involved in sugar uptake in bacteria. This transport process is dependent on several cytoplasmic phosphoryl transfer proteins - Enzyme I (I), HPr, Enzyme IIA (IIA), and Enzyme IIB (IIB) as well as the integral membrane sugar permease complex (IICD). It is not part of the PTS-AG or PTS-GFL superfamilies.