
Cellulase is any of several enzymes produced chiefly by fungi, bacteria, and protozoans that catalyze cellulolysis, the decomposition of cellulose and of some related polysaccharides:

Acarbose (INN) is an anti-diabetic drug used to treat diabetes mellitus type 2 and, in some countries, prediabetes. It is a generic sold in Europe and China as Glucobay, in North America as Precose, and in Canada as Prandase. It is cheap and popular in China, but not in the U.S. One physician explains that use in the U.S. is limited because it is not potent enough to justify the side effects of diarrhea and flatulence. However, a large study concluded in 2013 that "acarbose is effective, safe and well tolerated in a large cohort of Asian patients with type 2 diabetes." A possible explanation for the differing opinions is an observation that acarbose is significantly more effective in patients eating a relatively high carbohydrate Eastern diet.
Alpha-glucosidase inhibitors (AGIs) are oral anti-diabetic drugs used for diabetes mellitus type 2 that work by preventing the digestion of carbohydrates. Carbohydrates are normally converted into simple sugars (monosaccharides) by alpha-glucosidase enzymes present on cells lining the intestine, enabling monosaccharides to be absorbed through the intestine. Hence, alpha-glucosidase inhibitors reduce the impact of dietary carbohydrates on blood sugar.

α-Glucosidase is a glucosidase located in the brush border of the small intestine that acts upon α(1→4) bonds:

Glycoside hydrolases catalyze the hydrolysis of glycosidic bonds in complex sugars. They are extremely common enzymes with roles in nature including degradation of biomass such as cellulose (cellulase), hemicellulose, and starch (amylase), in anti-bacterial defense strategies, in pathogenesis mechanisms and in normal cellular function. Together with glycosyltransferases, glycosidases form the major catalytic machinery for the synthesis and breakage of glycosidic bonds.
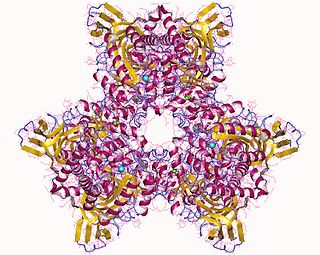
Atrazine Chlorohydrolase (AtzA) is an enzyme (E.C.3.8.1.8), which catalyzes the conversion of atrazine to hydroxyatrazine. Bacterial degradation determines the environmental impact and efficacy of an herbicide or pesticide. Initially, most pesticides are highly effective and show minimal bacterial degradation; however, bacteria can rapidly evolve and gain the ability to metabolize potential nutrients in the environment. Despite a remarkable structural similarity, degradation of atrazine by bacteria capable of melamine degradation was rare; however, since its introduction as a pesticide in the United States, bacteria capable of atrazine degradation have evolved. Currently, Pseudomonas sp. strain ADP seems to be the optimal bacterial strain for atrazine degradations, which appears to be the sole nitrogen source for the bacteria.
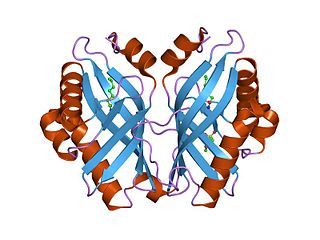
In enzymology, a limonene-1,2-epoxide hydrolase (EC 3.3.2.8) is an enzyme that catalyzes the chemical reaction
The enzyme 4-hydroxybenzoyl-CoA thioesterase (EC 3.1.2.23) catalyzes the reaction
The enzyme arylesterase (EC 3.1.1.2) catalyzes the reaction
The enzyme feruloyl esterase (EC 3.1.1.73) catalyzes the reaction
The enzyme pyridoxal phosphatase (EC 3.1.3.74) catalyzes the reaction
The enzyme tannase (EC 3.1.1.20) catalyzes the following reaction:

In enzymology, a glucosylceramidase (EC 3.2.1.45) is an enzyme that catalyzes the chemical reaction

In enzymology, an agmatinase (EC 3.5.3.11) is an enzyme that catalyzes the chemical reaction

In enzymology, a cyclomaltodextrin glucanotransferase is an enzyme that catalyzes the chemical reaction of cyclizing part of a 1,4-alpha-D-glucan molecule through the formation of a 1,4-alpha-D-glucosidic bond. They are bacterial enzymes belonging to the same family of the α-amylase specifically known as glycosyl-hydrolase family 13. This peculiar enzyme is capable of catalyzing more than one reaction with the most important being the synthesis of non-reducing cyclic dextrins known as cyclodextrins starting from starch, amylose, and other polysaccharides.

Acarviosin is a sugar composed of cyclohexitol linked to a 4-amino-4,6-dideoxy-D-glucopyranose. Acarviosin is part of the potent α-amylase inhibitor acarbose and its derivatives. Acarviosin is a product of the degradation of acarbose by gut microbiota, the glycoside hydrolase from gut bacteria Lactobacillus plantarum is able to hydrolyze acarbose to maltose and acarviosin. The nitrogen atom binds to α-amylase more tightly than the natural substrate making it more potent than other inhibitors. Several other acarviosin-containing α-amylase inhibitors have been found in microbes including isovalertatins and butytatins from Streptomyces luteogriseus and longer oligosaccharides from Streptomyces coelicoflavus.

(R)-prunasin is a cyanogenic glycoside related to amygdalin. Chemically, it is the glucoside of (R)-mandelonitrile.

Glucan 1,4-alpha-maltohydrolase is an enzyme with systematic name 4-alpha-D-glucan alpha-maltohydrolase. This enzyme catalyses the following chemical reaction
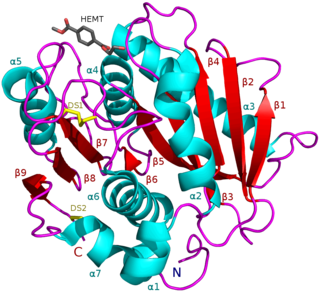
PETases are an esterase class of enzymes that catalyze the breakdown (via hydrolysis) of polyethylene terephthalate (PET) plastic to monomeric mono-2-hydroxyethyl terephthalate (MHET). The idealized chemical reaction is:
Cytophaga hutchinsonii is a bacterial species in the genus Cytophaga. C. hutchinsonii is an aerobic, gram-negative, soil, microorganism that exhibits gliding motility, enabling it to move quickly over surfaces and is capable of cellulose degradation.













