
γ-Aminobutyric acid, or GABA, is the chief inhibitory neurotransmitter in the developmentally mature mammalian central nervous system. Its principal role is reducing neuronal excitability throughout the nervous system.

The GABA receptors are a class of receptors that respond to the neurotransmitter gamma-aminobutyric acid (GABA), the chief inhibitory compound in the mature vertebrate central nervous system. There are two classes of GABA receptors: GABAA and GABAB. GABAA receptors are ligand-gated ion channels ; whereas GABAB receptors are G protein-coupled receptors, also called metabotropic receptors.
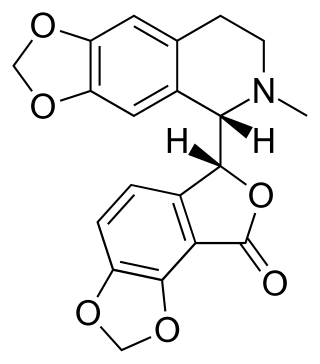
Bicuculline is a phthalide-isoquinoline compound that is a light-sensitive competitive antagonist of GABAA receptors. It was originally identified in 1932 in plant alkaloid extracts and has been isolated from Dicentra cucullaria, Adlumia fungosa, and several Corydalis species. Since it blocks the inhibitory action of GABA receptors, the action of bicuculline mimics epilepsy; it also causes convulsions. This property is utilized in laboratories around the world in the in vitro study of epilepsy, generally in hippocampal or cortical neurons in prepared brain slices from rodents. This compound is also routinely used to isolate glutamatergic receptor function.

CNQX or cyanquixaline (6-cyano-7-nitroquinoxaline-2,3-dione) is a competitive AMPA/kainate receptor antagonist. Its chemical formula is C9H4N4O4. CNQX is often used in the retina to block the responses of OFF-bipolar cells for electrophysiology recordings.

The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Upon opening, the GABAA receptor on the postsynaptic cell is selectively permeable to chloride ions (Cl−) and, to a lesser extent, bicarbonate ions (HCO3−). Depending on the membrane potential and the ionic concentration difference, this can result in ionic fluxes across the pore. If the membrane potential is higher than the equilibrium potential (also known as the reversal potential) for chloride ions, when the receptor is activated Cl− will flow into the cell. This causes an inhibitory effect on neurotransmission by diminishing the chance of a successful action potential occurring at the postsynaptic cell. The reversal potential of the GABAA-mediated inhibitory postsynaptic potential (IPSP) in normal solution is −70 mV, contrasting the GABAB IPSP (-100 mV).

The glycine receptor is the receptor of the amino acid neurotransmitter glycine. GlyR is an ionotropic receptor that produces its effects through chloride current. It is one of the most widely distributed inhibitory receptors in the central nervous system and has important roles in a variety of physiological processes, especially in mediating inhibitory neurotransmission in the spinal cord and brainstem.
GABAB receptors (GABABR) are G-protein coupled receptors for gamma-aminobutyric acid (GABA), therefore making them metabotropic receptors, that are linked via G-proteins to potassium channels. The changing potassium concentrations hyperpolarize the cell at the end of an action potential. The reversal potential of the GABAB-mediated IPSP is –100 mV, which is much more hyperpolarized than the GABAA IPSP. GABAB receptors are found in the central nervous system and the autonomic division of the peripheral nervous system.
The GABAA-rho receptor is a subclass of GABAA receptors composed entirely of rho (ρ) subunits. GABAA receptors including those of the ρ-subclass are ligand-gated ion channels responsible for mediating the effects of gamma-amino butyric acid (GABA), the major inhibitory neurotransmitter in the brain. The GABAA-ρ receptor, like other GABAA receptors, is expressed in many areas of the brain, but in contrast to other GABAA receptors, the GABAA-ρ receptor has especially high expression in the retina.

Gabazine (SR-95531) is a drug that acts as an antagonist at GABAA receptors. It is used in scientific research and has no role in medicine, as it would be expected to produce convulsions if used in humans.

Saclofen is a competitive antagonist for the GABAB receptor. This drug is an analogue of the GABAB agonist baclofen. The GABAB receptor is heptahelical receptor, expressed as an obligate heterodimer, which couples to the Gi/o class of heterotrimeric G-proteins. The action of saclofen on the central nervous system is understandably modest, because G-proteins rely on an enzyme cascade to alter cell behavior while ionotropic receptors immediately change the ionic permeability of the neuronal plasma membrane, thus changing its firing patterns. These particular receptors, presynaptically inhibit N- and P/Q- voltage-gated calcium channels (VGCCs) via a direct interaction of the dissociated beta gamma subunit of the g-protein with the intracellular loop between the 1st and 2nd domain of the VGCC's alpha-subunit; postsynaptically, these potentiate Kir currents. Both result in inhibitory effects.

Etazolate (SQ-20,009, EHT-0202) is an anxiolytic drug which is a pyrazolopyridine derivative and has unique pharmacological properties. It acts as a positive allosteric modulator of the GABAA receptor at the barbiturate binding site, as an adenosine antagonist of the A1 and A2 subtypes, and as a phosphodiesterase inhibitor selective for the PDE4 isoform. It is currently in clinical trials for the treatment of Alzheimer's disease.

Gamma-aminobutyric acid receptor subunit delta is a protein that in humans is encoded by the GABRD gene. In the mammalian brain, the delta (δ) subunit forms specific GABAA receptor subtypes by co-assembly leading to δ subunit containing GABAA receptors.
A convulsant is a drug which induces convulsions and/or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to the risk of convulsions and consequent excitotoxicity. Most convulsants are antagonists at either the GABAA or glycine receptors, or ionotropic glutamate receptor agonists. Many other drugs may cause convulsions as a side effect at high doses but only drugs whose primary action is to cause convulsions are known as convulsants. Nerve agents such as sarin, which were developed as chemical weapons, produce convulsions as a major part of their toxidrome, but also produce a number of other effects in the body and are usually classified separately. Dieldrin which was developed as an insecticide blocks chloride influx into the neurons causing hyperexcitability of the CNS and convulsions. The Irwin observation test and other studies that record clinical signs are used to test the potential for a drug to induce convulsions. Camphor, and other terpenes given to children with colds can act as convulsants in children who have had febrile seizures.
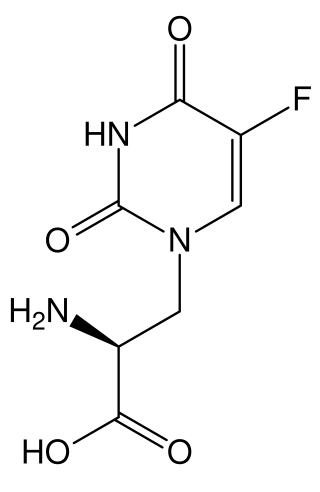
5-Fluorowillardiine is a selective agonist for the AMPA receptor, with only limited effects at the kainate receptor. It is an excitotoxic neurotoxin when used in vivo and so is rarely used in intact animals, but it is widely used to selectively stimulate AMPA receptors in vitro. It is structurally similar to the compound willardiine, which is also an agonist for the AMPA and kainate receptors. Willardiine occurs naturally in Mariosousa willardiana and Acacia sensu lato.

GYKI 52466 is a 2,3-benzodiazepine that acts as an ionotropic glutamate receptor antagonist, which is a non-competitive AMPA receptor antagonist (IC50 values are 10-20, ~ 450 and >> 50 μM for AMPA-, kainate- and NMDA-induced responses respectively), orally-active anticonvulsant, and skeletal muscle relaxant. Unlike conventional 1,4-benzodiazepines, GYKI 52466 and related 2,3-benzodiazepines do not act on GABAA receptors. Like other AMPA receptor antagonists, GYKI 52466 has anticonvulsant and neuroprotective properties.

Cartazolate (SQ-65,396) is a drug of the pyrazolopyridine class. It acts as a GABAA receptor positive allosteric modulator at the barbiturate binding site of the complex and has anxiolytic effects in animals. It is also known to act as an adenosine antagonist at the A1 and A2 subtypes and as a phosphodiesterase inhibitor. Cartazolate was tested in human clinical trials and was found to be efficacious for anxiety but was never marketed. It was developed by a team at E.R. Squibb and Sons in the 1970s.

Quisqualamine is the α-decarboxylated analogue of quisqualic acid, as well as a relative of the neurotransmitters glutamate and γ-aminobutyric acid (GABA). α-Decarboxylation of excitatory amino acids can produce derivatives with inhibitory effects. Indeed, unlike quisqualic acid, quisqualamine has central depressant and neuroprotective properties and appears to act predominantly as an agonist of the GABAA receptor and also to a lesser extent as an agonist of the glycine receptor, due to the facts that its actions are inhibited in vitro by GABAA antagonists like bicuculline and picrotoxin and by the glycine antagonist strychnine, respectively. Mg2+ and DL-AP5, NMDA receptor blockers, CNQX, an antagonist of both the AMPA and kainate receptors, and 2-hydroxysaclofen, a GABAB receptor antagonist, do not affect quisqualamine's actions in vitro, suggesting that it does not directly affect the ionotropic glutamate receptors or the GABAB receptor in any way. Whether it binds to and acts upon any of the metabotropic glutamate receptors like its analogue quisqualic acid however is unclear.

HA-966 or (±) 3-Amino-1-hydroxy-pyrrolidin-2-one is a molecule used in scientific research as a glycine receptor and NMDA receptor antagonist / low efficacy partial agonist. It has neuroprotective and anticonvulsant, anxiolytic, antinociceptive and sedative / hypnotic effects in animal models. Pilot human clinical trials in the early 1960s showed that HA-966 appeared to benefit patients with tremors of extrapyramidal origin.

In pharmacology, GABAA receptor positive allosteric modulators, also known as GABAkines or GABAA receptor potentiators, are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.

IPTBO is a bicyclic phosphate convulsant. It is an extremely potent GABA receptor antagonist that can cause violent convulsions in mice.

















