
Pharmacology is a branch of medicine, biology and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous molecule which exerts a biochemical or physiological effect on the cell, tissue, organ, or organism. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.

The Merck Index is an encyclopedia of chemicals, drugs and biologicals with over 10,000 monograph on single substances or groups of related compounds published online by the Royal Society of Chemistry.
In the physical sciences, a partition coefficient (P) or distribution coefficient (D) is the ratio of concentrations of a compound in a mixture of two immiscible solvents at equilibrium. This ratio is therefore a comparison of the solubilities of the solute in these two liquids. The partition coefficient generally refers to the concentration ratio of un-ionized species of compound, whereas the distribution coefficient refers to the concentration ratio of all species of the compound.
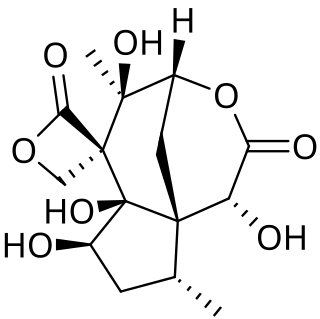
Forensic toxicology is the use of toxicology and disciplines such as analytical chemistry, pharmacology and clinical chemistry to aid medical or legal investigation of death, poisoning, and drug use. The primary concern for forensic toxicology is not the legal outcome of the toxicological investigation or the technology utilized, but rather the obtainment and interpretation of results. A toxicological analysis can be done to various kinds of samples. A forensic toxicologist must consider the context of an investigation, in particular any physical symptoms recorded, and any evidence collected at a crime scene that may narrow the search, such as pill bottles, powders, trace residue, and any available chemicals. Provided with this information and samples with which to work, the forensic toxicologist must determine which toxic substances are present, in what concentrations, and the probable effect of those chemicals on the person.

Butriptyline, sold under the brand name Evadyne among others, is a tricyclic antidepressant (TCA) that has been used in the United Kingdom and several other European countries for the treatment of depression but appears to no longer be marketed. Along with trimipramine, iprindole, and amoxapine, it has been described as an "atypical" or "second-generation" TCA due to its relatively late introduction and atypical pharmacology. It was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.

Protriptyline, sold under the brand name Vivactil among others, is a tricyclic antidepressant (TCA), specifically a secondary amine, indicated for the treatment of depression and attention-deficit hyperactivity disorder (ADHD). Uniquely among most of the TCAs, protriptyline tends to be energizing instead of sedating, and is sometimes used for narcolepsy to achieve a wakefulness-promoting effect.

Melitracen is a tricyclic antidepressant (TCA), for the treatment of depression and anxiety. In addition to single drug preparations, it is also available as Deanxit, marketed by Lundbeck, a combination product containing both melitracen and flupentixol.

ChemSpider is a database of chemicals. ChemSpider is owned by the Royal Society of Chemistry.

Cloperastine (INN) or cloperastin, also known as cloperastine hydrochloride (JAN) and cloperastine fendizoate, is an antitussive and antihistamine that is marketed as a cough suppressant in Japan, Hong Kong, and in some European countries. It was first introduced in 1972 in Japan, and then in Italy in 1981.
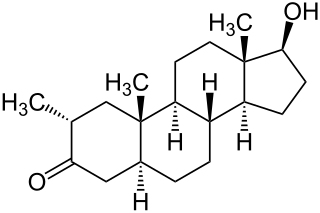
Drostanolone, or dromostanolone, is an anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which was never marketed. An androgen ester prodrug of drostanolone, drostanolone propionate, was formerly used in the treatment of breast cancer in women under brand names such as Drolban, Masteril, and Masteron. This has also been used non-medically for physique- or performance-enhancing purposes.

Oxaflozane (INN) is an antidepressant and anxiolytic drug that was introduced by Solvay in France in 1982 for the treatment of depression but has since been discontinued. It is a prodrug of flumexadol, which is reported to act as an agonist of the serotonin 5-HT1A and 5-HT2C receptors and, to a much lesser extent, of the 5-HT2A receptor. In addition to its serotonergic properties, oxaflozane may also produce anticholinergic side effects at high doses, namely in overdose.
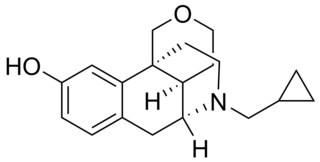
Proxorphan (INN), also known as proxorphan tartate (USAN), is an opioid analgesic and antitussive drug of the morphinan family that was never marketed. It acts preferentially as a κ-opioid receptor partial agonist and to a lesser extent as a μ-opioid receptor partial agonist.

Isomethadone is a synthetic opioid analgesic and antitussive related to methadone that was used formerly as a pharmaceutical drug but is now no longer marketed. Isomethadone was used as both an analgesic and antitussive. It binds to and activates both the μ- and δ-opioid receptors, with the (S)-isomer being the more potent of its two enantiomers. Isomethadone is a Schedule II controlled substance in the United States, with an ACSCN of 9226 and a 2014 aggregate manufacturing quota of 5 g. The salts in use are the hydrobromide, hydrochloride, and HCl monohydrate (0.850). Isomethadone is also regulated internationally as a Schedule I controlled substance under the United Nations Single Convention on Narcotic Drugs of 1961.

Megestrol is a progestin of the 17α-hydroxyprogesterone group which was never marketed and is not currently used clinically. Its acylated derivative megestrol acetate is also a progestogen, which, in contrast to megestrol itself, has been extensively used as a pharmaceutical drug.

Penmesterol (INN), or penmestrol, also known as 17α-methyltestosterone 3-cyclopentyl enol ether, is a synthetic, orally active anabolic-androgenic steroid (AAS) that was developed in the early 1960s. It is the 3-cyclopentyl enol ether of methyltestosterone.
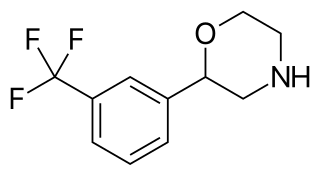
Flumexadol (INN) is a drug described and researched as a non-opioid analgesic which was never marketed. It has been found to act as an agonist of the serotonin 5-HT1A and 5-HT2C receptors and, to a much lesser extent, of the 5-HT2A receptor. According to Nilsson (2006) in a paper on 5-HT2C receptor agonists as potential anorectics, "The (+)-enantiomer of this compound showed [...] affinity for the 5-HT2C receptor (Ki) 25 nM) [...] and was 40-fold selective over the 5-HT2A receptor in receptor binding studies. Curiously, the racemic version [...], also known as 1841 CERM, was originally reported to possess analgesic properties while no association with 5-HT2C receptor activity was mentioned." It is implied that flumexadol might be employable as an anorectic in addition to analgesic. Though flumexadol itself has never been approved for medical use, oxaflozane is a prodrug of the compound that was formerly used clinically in France as an antidepressant and anxiolytic agent.

Bisdehydrodoisynolic acid (BDDA), as the (Z)-isomer ( -BDDA), is a synthetic, nonsteroidal estrogen related to doisynolic acid that was never marketed. It is one of the most potent estrogens known, although it has more recently been characterized as a selective estrogen receptor modulator (SERM). BDDA and other doisynolic acid derivatives display relatively low affinity accompanied by disproportionately high estrogenic potency in vivo, which was eventually determined to be due to transformation into metabolites with greater estrogenic activity. The drug was discovered in 1947 as a degradation product of the reaction of equilenin or dihydroequilenin with potassium hydroxide. It is the seco-analogue of equilenin, while doisynolic acid is the seco-analogue of estrone. These compounds, along with diethylstilbestrol, can be considered to be open-ring analogues of estradiol. The methyl ether of BDDA, doisynoestrol, is also an estrogen, and in contrast to BDDA, has been marketed.

6,6-Difluoronorethisterone, also known as 6,6-difluoro-17α-ethynyl-19-nortestosterone or as 6,6-difluoro-17α-ethynylestr-4-en-17β-ol-3-one, is a steroidal progestin of the 19-nortestosterone group that was described in 1971 but was never marketed. It is a fluorinated derivative of norethisterone. The C17β acetate ester, 6,6-difluoronorethisterone acetate, has also been synthesized and described.

Prednisolamate, also known as prednisolone 21-diethylaminoacetate, is a synthetic corticosteroid. It is or was a component of Etaproctene, which contains lidocaine, prednisolamate hydrochloride, and tetryzoline.

Triamcinolone aminobenzal benzamidoisobutyrate is a synthetic glucocorticoid corticosteroid which is no longer marketed.



















