
Ethylene glycol is an organic compound with the formula (CH2OH)2. It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. It has a sweet taste, but is toxic in high concentrations. This molecule has been observed in outer space.

Ethylene oxide is an organic compound with the formula C2H4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of a silver catalyst.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting groups of carbonyl groups, making them essential in synthesis of organic chemistry.

Malonic acid is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word μᾶλον (malon) meaning 'apple'.

Polyglycolide or poly(glycolic acid) (PGA), also spelled as polyglycolic acid, is a biodegradable, thermoplastic polymer and the simplest linear, aliphatic polyester. It can be prepared starting from glycolic acid by means of polycondensation or ring-opening polymerization. PGA has been known since 1954 as a tough fiber-forming polymer. Owing to its hydrolytic instability, however, its use has initially been limited. Currently polyglycolide and its copolymers (poly(lactic-co-glycolic acid) with lactic acid, poly(glycolide-co-caprolactone) with ε-caprolactone and poly (glycolide-co-trimethylene carbonate) with trimethylene carbonate) are widely used as a material for the synthesis of absorbable sutures and are being evaluated in the biomedical field.

Glutaric acid is the organic compound with the formula C3H6(COOH)2. Although the related "linear" dicarboxylic acids adipic and succinic acids are water-soluble only to a few percent at room temperature, the water-solubility of glutaric acid is over 50% (w/w).
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups. The general molecular formula for dicarboxylic acids can be written as HO2C−R−CO2H, where R can be aliphatic or aromatic. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids.

Aleksandr Pavlovich Dianin was a Russian chemist from Saint Petersburg. He carried out studies on phenols and discovered a phenol derivative now known as bisphenol A and the accordingly named Dianin's compound. He was married to the adopted daughter of fellow chemist Alexander Borodin. In 1887, Dianin succeeded his father-in-law as chair of the Chemistry Department at the Imperial Medical-Surgical Academy in St. Petersburg.

Wilhelm Rudolph Fittig was a German chemist. He discovered the pinacol coupling reaction, mesitylene, diacetyl and biphenyl. Fittig studied the action of sodium on ketones and hydrocarbons. He discovered the Fittig reaction or Wurtz–Fittig reaction for the synthesis of alkylbenzenes, he proposed a diketone structure for benzoquinone and isolated phenanthrene from coal tar. He discovered and synthesized the first lactones and investigated structures of piperine, naphthalene, and fluorene.

In organic chemistry, a carbonate ester is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R−O−C(=O)−O−R' and they are related to esters, ethers and also to the inorganic carbonates.

Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
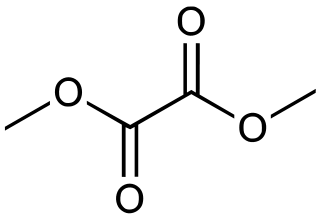
Dimethyl oxalate is an organic compound with the formula (CO2CH3)2 or (CH3)2C2O4. It is the dimethyl ester of oxalic acid. Dimethyl oxalate is a colorless or white solid that is soluble in water.

Phenylacetaldehyde is an organic compound used in the synthesis of fragrances and polymers. Phenylacetaldehyde is an aldehyde that consists of acetaldehyde bearing a phenyl substituent; the parent member of the phenylacetaldehyde class of compounds. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is an alpha-CH2-containing aldehyde and a member of phenylacetaldehydes.
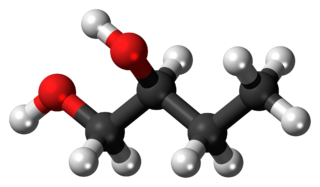
1,2-Butanediol is the organic compound with the formula HOCH2(HO)CHCH2CH3. It is classified as a vic-diol (glycol). It is chiral, although typically it is encountered as the racemic mixture. It is a colorless liquid.

Polybutylene succinate (PBS) is a thermoplastic polymer resin of the polyester family. PBS is a biodegradable aliphatic polyester with properties that are comparable to polypropylene.

2-Methylglutaronitrile is the organic compound with the formula NCCH2CH2CH(CH3)CN. This dinitrile is obtained in the large-scale synthesis of adiponitrile. It is a colorless liquid with an unpleasant odor. It is the starting compound for the vitamin nicotinamide and for the diester dimethyl-2-methylglutarate and the ester amide methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate, which are promoted as green solvents. 2-Methylglutaronitrile is chiral but is mainly encountered as the racemate. It is also used to make Dytek A.
p-Dioxanone (1,4-dioxan-2-one) is the lactone of 2-(2-hydroxyethoxy)acetic acid. It is a monomer that can undergo ring-opening polymerization to give polydioxanone, a biodegradable implant material. It is isomeric to trimethylene carbonate (1,3-dioxan-2-one).
1-Vinylimidazole is a water-soluble basic monomer that forms quaternizable homopolymers by free-radical polymerization with a variety of vinyl and acrylic monomers. The products are functional copolymers, which are used as oil field chemicals and as cosmetic auxiliaries. 1-Vinylimidazole acts as a reactive diluent in UV lacquers, inks, and adhesives.
1,4-butane sultone is a six-membered δ-sultone and the cyclic ester of 4-hydroxybutanesulfonic acid. As a sulfo-alkylating agent, 1,4-butanesultone is used to introduce the sulfobutyl group (–(CH2)4–SO3−) into hydrophobic compounds possessing nucleophilic functional groups, for example hydroxy groups (as in the case of β-cyclodextrin) or amino groups (as in the case of polymethine dyes). In such, the sulfobutyl group is present as neutral sodium salt and considerably increases the water solubility of the derivatives.
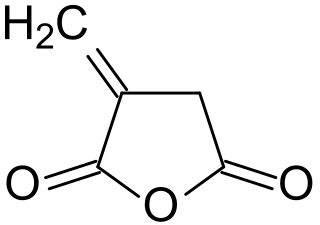
Itaconic anhydride is the cyclic anhydride of itaconic acid and is obtained by the pyrolysis of citric acid. It is a colourless, crystalline solid, which dissolves in many polar organic solvents and hydrolyzes forming itaconic acid. Itaconic anhydride and its derivative itaconic acid have been promoted as biobased "platform chemicals" and bio- building blocks.) These expectations, however, have not been fulfilled.




















