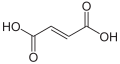
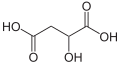
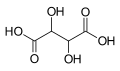
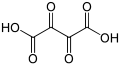
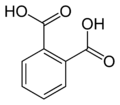
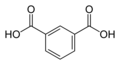
Partial list of saturated dicarboxylic acids
Some common or illustrative examples
C n Common name Systematic IUPAC name Structure pKa1 pKa2 PubChem C2 0 Oxalic acid ethanedioic acid 
1.27 4.27 971 C3 1 Malonic acid propanedioic acid 
2.85 5.05 867 C4 2 Succinic acid butanedioic acid 
4.21 5.41 1110 C5 3 Glutaric acid pentanedioic acid 
4.34 5.41 743 C6 4 Adipic acid hexanedioic acid 
4.41 5.41 196 C7 5 Pimelic acid heptanedioic acid 
4.50 5.43 385 C8 6 Suberic acid octanedioic acid 
4.526 5.498 10457 C8 6 1,4-Cyclohexanedicarboxylic acid 
14106 C9 7 Azelaic acid nonanedioic acid 
4.550 5.498 2266 C10 8 Sebacic acid decanedioic acid 
4.720 5.450 5192 C11 9 undecanedioic acid 
15816 C12 10 dodecanedioic acid 
12736 C13 11 Brassylic acid tridecanedioic acid 
10458 C16 14 Thapsic acid hexadecanedioic acid 
10459 C21 19 Japanic acid heneicosanedioic acid 
9543668 C22 20 Phellogenic acid docosanedioic acid 
244872 C30 28 Equisetolic acid triacontanedioic acid 
5322010