
A cell wall is a structural layer that surrounds some cell types, found immediately outside the cell membrane. It can be tough, flexible, and sometimes rigid. Primarily, it provides the cell with structural support, shape, protection, and functions as a selective barrier. Another vital role of the cell wall is to help the cell withstand osmotic pressure and mechanical stress. While absent in many eukaryotes, including animals, cell walls are prevalent in other organisms such as fungi, algae and plants, and are commonly found in most prokaryotes, with the exception of mollicute bacteria.

Bark is the outermost layer of stems and roots of woody plants. Plants with bark include trees, woody vines, and shrubs. Bark refers to all the tissues outside the vascular cambium and is a nontechnical term. It overlays the wood and consists of the inner bark and the outer bark. The inner bark, which in older stems is living tissue, includes the innermost layer of the periderm. The outer bark on older stems includes the dead tissue on the surface of the stems, along with parts of the outermost periderm and all the tissues on the outer side of the periderm. The outer bark on trees which lies external to the living periderm is also called the rhytidome.

Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity and do not rot easily. Chemically, lignins are polymers made by cross-linking phenolic precursors.

Hardwood is wood from angiosperm trees. These are usually found in broad-leaved temperate and tropical forests. In temperate and boreal latitudes they are mostly deciduous, but in tropics and subtropics mostly evergreen. Hardwood contrasts with softwood.

Teichoic acids are bacterial copolymers of glycerol phosphate or ribitol phosphate and carbohydrates linked via phosphodiester bonds.

The endodermis is the innermost layer of cortex in land plants. It is a cylinder of compact living cells, the radial walls of which are impregnated with hydrophobic substances to restrict apoplastic flow of water to the inside. The endodermis is the boundary between the cortex and the stele.

The Casparian strip is a band-like thickening in the center of the root endodermis of vascular plants. The composition of the region is mainly suberin, lignin and some structural proteins, which are capable of reducing the diffusive apoplastic flow of water and solutes into the stele and its width varies between species. The Casparian strip is impervious to water so can control the transportation of water and inorganic salts between the cortex and the vascular bundle, preventing water and inorganic salts from being transported to the stele through the apoplast, so that it must enter the cell membrane and move to the stele through the symplastic pathway, blocking the internal and external objects of the cell. The function of mass transportation are similar to that of animal tissues.. The development of the Casparian strip is regulated by transcription factors such as SHORT-ROOT (SHR), SCARECROW (SCR) and MYB36, as well as polypeptide hormone synthesised by midcolumn cells.
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups. The general molecular formula for dicarboxylic acids can be written as HO2C−R−CO2H, where R can be aliphatic or aromatic. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids.

Abscission is the shedding of various parts of an organism, such as a plant dropping a leaf, fruit, flower, or seed. In zoology, abscission is the intentional shedding of a body part, such as the shedding of a claw, husk, or the autotomy of a tail to evade a predator. In mycology, it is the liberation of a fungal spore. In cell biology, abscission refers to the separation of two daughter cells at the completion of cytokinesis.

Ferulic acid is a hydroxycinnamic acid; it is an organic compound with the formula (CH3O)HOC6H3CH=CHCO2H. The name is derived from the genus Ferula, referring to the giant fennel (Ferula communis). Classified as a phenolic phytochemical, ferulic acid is an amber colored solid. Esters of ferulic acid are found in plant cell walls, covalently bonded to hemicellulose such as arabinoxylans. Salts and esters derived from ferulic acid are called ferulates.

An appressorium is a specialized cell typical of many fungal plant pathogens that is used to infect host plants. It is a flattened, hyphal "pressing" organ, from which a minute infection peg grows and enters the host, using turgor pressure capable of punching through even Mylar.

Coniferyl alcohol is an organic compound with the formula HO(CH3O)C6H3CH=CHCH2OH. A colourless or white solid, it is one of the monolignols, produced via the phenylpropanoid biochemical pathway. When copolymerized with related aromatic compounds, coniferyl alcohol forms lignin or lignans. Coniferin is a glucoside of coniferyl alcohol. Coniferyl alcohol is an intermediate in biosynthesis of eugenol and of stilbenoids and coumarin. Gum benzoin contains significant amount of coniferyl alcohol and its esters. It is found in both gymnosperm and angiosperm plants. Sinapyl alcohol and paracoumaryl alcohol, the other two lignin monomers, are found in angiosperm plants and grasses.

The phenylpropanoids are a diverse family of organic compounds that are biosynthesized by plants from the amino acids phenylalanine and tyrosine in the shikimic acid pathway. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols, flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. The coumaroyl component is produced from cinnamic acid.

Lignocellulose refers to plant dry matter (biomass), so called lignocellulosic biomass. It is the most abundantly available raw material on the Earth for the production of biofuels. It is composed of two kinds of carbohydrate polymers, cellulose and hemicellulose, and an aromatic-rich polymer called lignin. Any biomass rich in cellulose, hemicelluloses, and lignin are commonly referred to as lignocellulosic biomass. Each component has a distinct chemical behavior. Being a composite of three very different components makes the processing of lignocellulose challenging. The evolved resistance to degradation or even separation is referred to as recalcitrance. Overcoming this recalcitrance to produce useful, high value products requires a combination of heat, chemicals, enzymes, and microorganisms. These carbohydrate-containing polymers contain different sugar monomers and they are covalently bound to lignin.
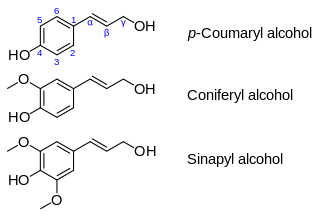
Monolignols, also called lignols, are the source materials for biosynthesis of both lignans and lignin and consist mainly of paracoumaryl alcohol (H), coniferyl alcohol (G) and sinapyl alcohol (S). These monolignols differ in their degree of methoxilation of the aromatic ring.

A plant cuticle is a protecting film covering the outermost skin layer (epidermis) of leaves, young shoots and other aerial plant organs that have no periderm. The film consists of lipid and hydrocarbon polymers infused with wax, and is synthesized exclusively by the epidermal cells.

The enzyme cutinase is a member of the hydrolase family. It catalyzes the following reaction:
Omega hydroxy acids are a class of naturally occurring straight-chain aliphatic organic acids n carbon atoms long with a carboxyl group at position 1, and a hydroxyl at terminal position n where n > 3. They are a subclass of hydroxycarboxylic acids. The C16 and C18 omega hydroxy acids 16-hydroxy palmitic acid and 18-hydroxy stearic acid are key monomers of cutin in the plant cuticle. The polymer cutin is formed by interesterification of omega hydroxy acids and derivatives of them that are substituted in mid-chain, such as 10,16-dihydroxy palmitic acid. Only the epidermal cells of plants synthesize cutin.

The exodermis is a physiological barrier that has a role in root function and protection. The exodermis is a membrane of variable permeability responsible for the radial flow of water, ions, and nutrients. It is the outer layer of a plant's cortex. The exodermis serves a double function as it can protect the root from invasion by foreign pathogens and ensures that the plant does not lose too much water through diffusion through the root system and can properly replenish its stores at an appropriate rate.
Juniperic acid or 16-hydroxyhexadecanoic acid is an omega-hydroxy long-chain fatty acid that is palmitic acid which is substituted at position 16 by a hydroxy group. Palmitic acid is converted to juniperic acid by cytochrome P450 various enzymes, including CYP704B22.

















