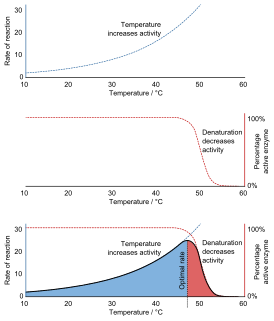
Denaturation is a process in which proteins or nucleic acids lose the quaternary structure, tertiary structure, and secondary structure which is present in their native state, by application of some external stress or compound such as a strong acid or base, a concentrated inorganic salt, an organic solvent, agitation and radiation or heat. If proteins in a living cell are denatured, this results in disruption of cell activity and possibly cell death. Protein denaturation is also a consequence of cell death. Denatured proteins can exhibit a wide range of characteristics, from conformational change and loss of solubility to aggregation due to the exposure of hydrophobic groups. The loss of solubility as a result of denaturation is called coagulation. Denatured proteins lose their 3D structure and therefore cannot function.

Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). If the sugar is ribose, the polymer is RNA; if the sugar is the ribose derivative deoxyribose, the polymer is DNA.
Pyrimidine is an aromatic heterocyclic organic compound similar to pyridine. One of the three diazines, it has the nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine. In nucleic acids, three types of nucleobases are pyrimidine derivatives: cytosine (C), thymine (T), and uracil (U).
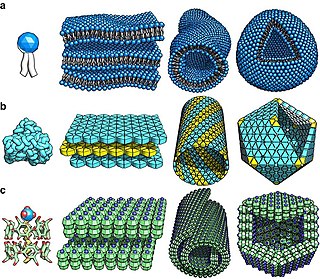
Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the constitutive components are molecules, the process is termed molecular self-assembly.
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.
Chemical biology is a scientific discipline spanning the fields of chemistry and biology. The discipline involves the application of chemical techniques, analysis, and often small molecules produced through synthetic chemistry, to the study and manipulation of biological systems. In contrast to biochemistry, which involves the study of the chemistry of biomolecules and regulation of biochemical pathways within and between cells, chemical biology deals with chemistry applied to biology.

A molecular machine, nanite, or nanomachine is a molecular component that produces quasi-mechanical movements (output) in response to specific stimuli (input). In cellular biology, macromolecular machines frequently perform tasks essential for life, such as DNA replication and ATP synthesis. The expression is often more generally applied to molecules that simply mimic functions that occur at the macroscopic level. The term is also common in nanotechnology where a number of highly complex molecular machines have been proposed that are aimed at the goal of constructing a molecular assembler.

Aptamers (APT-uh-murz) are short sequences of artificial DNA or RNA that bind a specific target molecule. Like antibodies, which are used for similar purposes in biotechnology and medicine, they can show strong binding to their target, with little or no off-target binding. To highlight how they are similar to and different from antibodies, aptamers are sometimes called “chemical antibodies.” Aptamers and antibodies can be used in many of the same tasks, but the nucleic acid-based structure of aptamers, which are mostly oligonucleotides, is very different from the amino acid-based structure of antibodies, which are proteins. This difference can make aptamers a better choice than antibodies for some purposes.
Stuart L. Schreiber is a scientist at Harvard University and co-Founder of the Broad Institute. He has been active in chemical biology, especially the use of small molecules as probes of biology and medicine. Small molecules are the molecules of life most associated with dynamic information flow; these work in concert with the macromolecules that are the basis for inherited information flow.

Khellin has been used as an herbal folk medicine, with use in the Mediterranean dating back to Ancient Egypt, to treat a variety of maladies including: renal colic, kidney stones, coronary disease, bronchial asthma, vitiligo, and psoriasis. It is a major constituent of the plant Ammi visnaga, also known as Bishop's Weed. Once purified, khellin exists as colorless, odorless, bitter-tasting needle-shaped crystals and is classified as a gamma-pyrone, a furanochromone derivative. In the early 20th century, researchers searched for khellin analogs with lower toxicity and better efficacy. A number of drugs were discovered through this research, such as amiodarone and cromolyn sodium, which are used in current medical practice. Efloxate is also mentioned as analog.
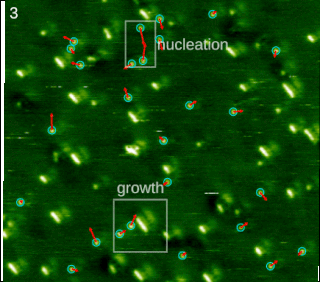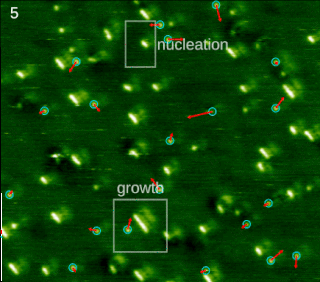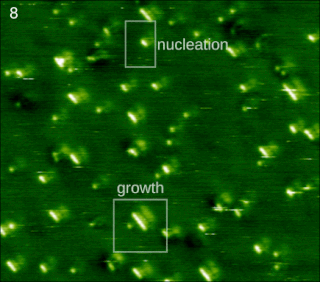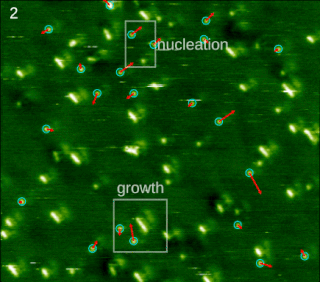
Molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly. These are intramolecular self-assembly and intermolecular self-assembly. Commonly, the term molecular self-assembly refers to intermolecular self-assembly, while the intramolecular analog is more commonly called folding.
Molecular binding is an attractive interaction between two molecules that results in a stable association in which the molecules are in close proximity to each other. It is formed when atoms or molecules bind together by sharing of electrons. It often, but not always, involves some chemical bonding.
A halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity. Like a hydrogen bond, the result is not a formal chemical bond, but rather a strong electrostaticattraction. Mathematically, the interaction can be decomposed in two terms: one describing an electrostatic, orbital-mixing charge-transfer and another describing electron-cloud dispersion. Halogen bonds find application in supramolecular chemistry; drug design and biochemistry; crystal engineering and liquid crystals; and organic catalysis.

Visnaga daucoides is a species of flowering plant in the carrot family known by many common names, including toothpick-plant, toothpickweed, bisnaga, khella, or sometimes bishop's weed. It is native to Europe, Asia, and North Africa, but it can be found throughout the world as an introduced species. This is an erect annual plant growing from a taproot to a maximum height near 80 centimeters. The leaves are up to 20 centimeters long and generally oval to triangular in shape but dissected into many small linear to lance-shaped segments. The inflorescence is a compound umbel of white flowers similar to those of other Apiaceae species. The fruit is a compressed oval-shaped body less than 3 millimeters long. This species is a source of khellin, a diuretic extract.

Nutlins are cis-imidazoline analogs which inhibit the interaction between mdm2 and tumor suppressor p53, and which were discovered by screening a chemical library by Vassilev et al. Nutlin-1, nutlin-2, and nutlin-3 were all identified in the same screen; however, Nutlin-3 is the compound most commonly used in anti-cancer studies. Nutlin small molecules occupy p53 binding pocket of MDM2 and effectively disrupt the p53–MDM2 interaction that leads to activation of the p53 pathway in p53 wild-type cells. Inhibiting the interaction between mdm2 and p53 stabilizes p53, and is thought to selectively induce a growth-inhibiting state called senescence in cancer cells. These compounds are therefore thought to work best on tumors that contain normal or "wild-type" p53. Nutlin-3 has been shown to affect the production of p53 within minutes.
Pimpinella monoica is now listed as a synonym for Pimpinella wallichiana(Miq.) Gandhi a plant species belonging to the genus Pimpinella.
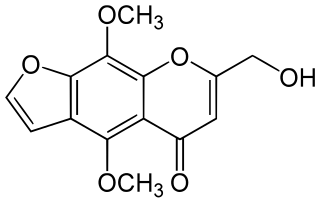
Ammiol is a furanochromone that can be found in Pimpinella monoica.

Visnagin is an organic chemical compound with the molecular formula C13H10O4 It is a furanochromone, a compound derivative of chromone (1,4-benzopyrone) and furan.

SNAP-tag® is a self-labeling protein tag commercially available in various expression vectors. SNAP-tag is a 182 residues polypeptide that can be fused to any protein of interest and further specifically and covalently tagged with a suitable ligand, such as a fluorescent dye. Since its introduction, SNAP-tag has found numerous applications in biochemistry and for the investigation of the function and localisation of proteins and enzymes in living cells. Compared to the current standard labelling methods used in fluorescence microscopy, the use of SNAP-tag presents significant advantages. SNAP-tag® is a registered trademark of New England Biolabs, Inc.

Roeland J. M. Nolte is a Dutch chemist, known for his work in the fields of organic chemistry, biochemistry, polymer chemistry, and supramolecular chemistry. He is an emeritus Royal Netherlands of Arts and Sciences professor and an emeritus professor of Organic Chemistry at Radboud University in Nijmegen, The Netherlands. Currently, he holds a special chair, i.e. professor of Molecular Nanotechnology, at this university. Nolte is considered to be one of the pioneers of the field of supramolecular chemistry, which encompasses the design and synthesis of new chemical structures from low molecular weight compounds and biopolymers using so-called non-covalent interactions. He published many studies on supramolecular assembly and biomimetic catalysts, which find applications in the field of nanomaterials and medicine.













