 | |
| Names | |
|---|---|
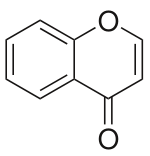
| IUPAC name Chromen-4-one | |
| Preferred IUPAC name 4H-1-Benzopyran-4-one | |
| Other names 4-Chromone; 1,4-Benzopyrone; 4H-Chromen-4-one; Benzo-gamma-pyrone; 1-Benzopyran-4-one; 4H-Benzo(b)pyran-4-one | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.035 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C9H6O2 | |
| Molar mass | 146.145 g·mol−1 |
| Acidity (pKa) | −2.0 (of conjugate acid) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Chromone (or 1,4-benzopyrone) is a derivative of benzopyran with a substituted keto group on the pyran ring. It is an isomer of coumarin.
Contents
Derivatives of chromone are collectively known as chromones. Most, though not all, chromones are also phenylpropanoids.