A molecular lesion, or a point lesion, is damage to the structure of a biological molecule such as DNA, RNA, or protein. This damage may result in the reduction or absence of normal function, and in rare cases the gain of a new function. Lesions in DNA may consist of breaks or other changes in chemical structure of the helix, ultimately preventing transcription. Meanwhile, lesions in proteins consist of both broken bonds and improper folding of the amino acid chain. While many nucleic acid lesions are general across DNA and RNA, some are specific to one, such as thymine dimers being found exclusively in DNA. Several cellular repair mechanisms exist, ranging from global to specific, in order to prevent lasting damage resulting from lesions.

Methylcholanthrene is a highly carcinogenic polycyclic aromatic hydrocarbon produced by burning organic compounds at very high temperatures. Methylcholanthrene is also known as 3-methylcholanthrene, 20-methylcholanthrene or the IUPAC name 3-methyl-1,2-dyhydrobenzo[j]aceanthrylene. The short notation often used is 3-MC or MCA. This compound forms pale yellow solid crystals when crystallized from benzene and ether. It has a melting point around 180 °C and its boiling point is around 280 °C at a pressure of 80 mmHg. Methylcholanthrene is used in laboratory studies of chemical carcinogenesis. It is an alkylated derivative of benz[a]anthracene and has a similar UV spectrum. The most common isomer is 3-methylcholanthrene, although the methyl group can occur in other places.

Psoralen is the parent compound in a family of naturally occurring organic compounds known as the linear furanocoumarins. It is structurally related to coumarin by the addition of a fused furan ring, and may be considered as a derivative of umbelliferone. Psoralen occurs naturally in the seeds of Psoralea corylifolia, as well as in the common fig, celery, parsley, West Indian satinwood, and in all citrus fruits. It is widely used in PUVA treatment for psoriasis, eczema, vitiligo, and cutaneous T-cell lymphoma; these applications are typically through the use of medications such as Methoxsalen. Many furanocoumarins are extremely toxic to fish, and some are deposited in streams in Indonesia to catch fish.

tert-Butylhydroquinone is a synthetic aromatic organic compound which is a type of phenol. It is a derivative of hydroquinone, substituted with a tert-butyl group.
4-Aminobiphenyl (4-APB) is an organic compound with the formula C6H5C6H4NH2. It is an amine derivative of biphenyl. It is a colorless solid, although aged samples can appear colored. 4-Aminobiphenyl was commonly used in the past as a rubber antioxidant and an intermediate for dyes. Exposure to this aryl-amine can happen through contact with chemical dyes and from inhalation of cigarette smoke. Researches showed that 4-aminobiphenyl is responsible for bladder cancer in humans and dogs by damaging DNA. Due to its carcinogenic effects, commercial production of 4-aminobiphenyl ceased in the United States in the 1950s.
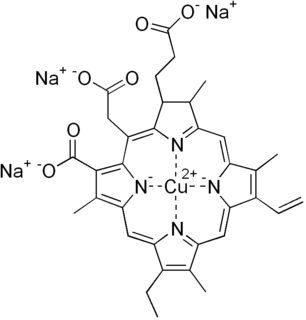
Chlorophyllin refers to any one of a group of closely related water-soluble salts that are semi-synthetic derivatives of chlorophyll, differing in the identity of the cations associated with the anion. Its most common form is a sodium/copper derivative used as a food additive and in alternative medicine. As a food coloring agent, copper complex chlorophyllin is known as natural green 3 and has the E number E141.

In molecular genetics, a DNA adduct is a segment of DNA bound to a cancer-causing chemical. This process could be the start of a cancerous cell, or carcinogenesis. DNA adducts in scientific experiments are used as biomarkers of exposure and as such are themselves measured to reflect quantitatively, for comparison, the amount of carcinogen exposure to the subject organism, for example rats or other living animals. Under experimental conditions for study, such DNA adducts are induced by known carcinogens, of which commonly used is DMBA. For example, the term "DMBA-DNA adduct" in a scientific journal refers to a piece of DNA that has DMBA attached to it. The presence of such an adduct indicates prior exposure to a potential carcinogen, but does not by itself indicate the presence of cancer in the subject animal.

The furanocoumarins, or furocoumarins, are a class of organic chemical compounds produced by a variety of plants. Most of the plant species found to contain furanocoumarins belong to a handful of plant families. The families Apiaceae and Rutaceae include the largest numbers of plant species that contain furanocoumarins. The families Moraceae and Fabaceae include a few, widely distributed plant species that contain furanocoumarins.

Bergamottin (5-geranoxypsoralen) is a natural furanocoumarin found in the pulp of pomelos and grapefruits. It is also found in the peel and pulp of the bergamot orange, from which it was first isolated and from which its name is derived.
Sodium salicylate is a sodium salt of salicylic acid. It can be prepared from sodium phenolate and carbon dioxide under higher temperature and pressure. Historically, it has been synthesized by refluxing methyl salicylate with an excess of sodium hydroxide.

N-Nitrosodiethylamine (NDEA) is an organic compound with the formula Et2NNO (Et = C2H5). A member of the nitrosamines, it is a light-sensitive, volatile, clear yellow oil that is soluble in water, lipids, and other organic solvents. It has an amine or aromatic odor. It is used as gasoline and lubricant additive, antioxidant, and stabilizer for industry materials. When heated to decomposition, N-nitrosodiethylamine emits toxic fumes of nitrogen oxides. N-Nitrosodiethylamine affects DNA integrity, probably by alkylation, and is used in experimental research to induce liver tumorigenesis. It is carcinogenic and mutagenic.
Tanning activators are chemicals that increase the effect of UV-radiation on the human skin.

Bergapten (5-methoxypsoralen) is a naturally-occurring organic chemical compound produced by numerous plant species, especially from the carrot family Apiaceae and the citrus family Rutaceae. For example, bergapten has been extracted from 24 species of the genus Heracleum in the family Apiaceae. In the family Rutaceae, various Citrus species contain significant amounts of bergapten, especially the bergamot orange, the micrantha, and certain varieties of lime and bitter orange.

Genistin is an isoflavone found in a number of dietary plants like soy and kudzu. It was first isolated in 1931 from the 90% methanol extract of a soybean meal, when it was found that hydrolysis with hydrochloric acid produced 1 mole each of genistein and glucose. Chemically it is the 7-O-beta-D-glucoside form of genistein and is the predominant form of the isoflavone naturally occurring in plants. In fact, studies in the 1970s revealed that 99% of the isoflavonoid compounds in soy are present as their glucosides. The glucosides are converted by digestive enzymes in the digestive system to exert their biological effects. Genistin is also converted to a more familiar genistein, thus, the biological activities including antiatherosclerotic, estrogenic and anticancer effects are analogous.

Caffeic acid phenethyl ester (CAPE) is a natural phenolic chemical compound. It is the ester of caffeic acid and phenethyl alcohol.
Arsenic biochemistry refers to biochemical processes that can use arsenic or its compounds, such as arsenate. Arsenic is a moderately abundant element in Earth's crust, and although many arsenic compounds are often considered highly toxic to most life, a wide variety of organoarsenic compounds are produced biologically and various organic and inorganic arsenic compounds are metabolized by numerous organisms. This pattern is general for other related elements, including selenium, which can exhibit both beneficial and deleterious effects. Arsenic biochemistry has become topical since many toxic arsenic compounds are found in some aquifers, potentially affecting many millions of people via biochemical processes.

Angelicin is the parent compound in a family of naturally occurring organic compounds known as the angular furanocoumarins. Structurally, it can be considered as benzapyra-2-one fused with a furan moiety in the 7,8-position. Angelicin is commonly found in certain Apiaceae and Fabaceae plant species such as Bituminaria bituminosa. It has a skin permeability coefficient (LogKp) of -2.46. The maximum absorption is observed at 300 nm. The 1HNMR spectrum is available; the infrared and mass spectra of angelicin can be found in this database. The sublimation of angelicin occurs at 120 °C and the pressure of 0.13 Pa. Angelicin is a coumarine.

Withaferin A is a steroidal lactone, derived from Acnistus arborescens, Withania somnifera and other members of family Solanaceae. It has been traditionally used in ayurvedic medicine. It is the first member of the withanolide class of ergostane type product to be discovered. This natural product has wide range of pharmacological activities including cardioprotective, anti-inflammatory, immuno-modulatory, anti-angiogenesis, anti-metastasis and anti-carcinogenic properties.
Antimutagens are the agents that interfere with the mutagenicity of a substance. The interference can be in the form of prevention of the transformation of a mutagenic compound into mutagen, inactivation, or otherwise the prevention of Mutagen-DNA reaction.
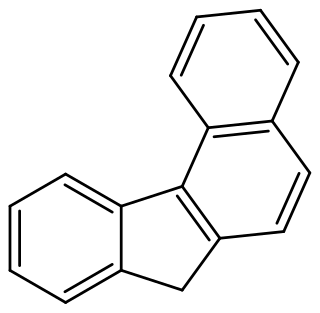
Benzo[c]fluorene is a polycyclic aromatic hydrocarbon (PAH) with mutagenic activity. It is a component of coal tar, cigarette smoke and smog and thought to be a major contributor to its carcinogenic properties. The mutagenicity of benzo[c]fluorene is mainly attributed to formation of metabolites that are reactive and capable of forming DNA adducts. According to the KEGG it is a group 3 carcinogen. Other names for benzo[c]fluorene are 7H-benzo[c]fluorene, 3,4-benzofluorene, and NSC 89264.
















