
Acetoacetic acid is the organic compound with the formula CH3COCH2COOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes. Acetoacetic acid is a weak acid.

Liquorice or licorice is the common name of Glycyrrhiza glabra, a flowering plant of the bean family Fabaceae, from the root of which a sweet, aromatic flavouring can be extracted.
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.

Paraxanthine, or 1,7-dimethylxanthine, is a dimethyl derivative of xanthine, structurally related to caffeine.

Homogentisic acid is a phenolic acid usually found in Arbutus unedo (strawberry-tree) honey. It is also present in the bacterial plant pathogen Xanthomonas campestris pv. phaseoli as well as in the yeast Yarrowia lipolytica where it is associated with the production of brown pigments. It is oxidatively dimerised to form hipposudoric acid, one of the main constituents of the 'blood sweat' of hippopotamuses.

Malvidin is an O-methylated anthocyanidin, the 3',5'-methoxy derivative of delphinidin. As a primary plant pigment, its glycosides are highly abundant in nature.
CapZ, also known as CAPZ, CAZ1 and CAPPA1, is a capping protein that caps the barbed end of actin filaments in muscle cells.
In enzymology, a scopoletin glucosyltransferase is an enzyme that catalyzes the chemical reaction

Ileal sodium/bile acid cotransporter, also known as apical sodium–bile acid transporter (ASBT) and ileal bile acid transporter (IBAT), is a bile acid:sodium symporter protein that in humans is encoded by the SLC10A2 gene.
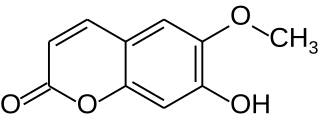
Scopoletin is a coumarin found in the root of plants in the genus Scopolia such as Scopolia carniolica and Scopolia japonica, in chicory, in Artemisia scoparia, in the roots and leaves of stinging nettle, in the passion flower, in Brunfelsia, in Viburnum prunifolium, in Solanum nigrum, in Datura metel, in Mallotus resinosus, or and in Kleinhovia hospita. It can also be found in fenugreek, vinegar, some whiskies or in dandelion coffee. A similar coumarin is scoparone. Scopoletin is highly fluorescent when dissolved in DMSO or water and is regularly used as a fluorimetric assay for the detection of hydrogen peroxide in conjunction with horseradish peroxidase. When oxidized, its fluorescence is strongly suppressed.

Angelicin is the parent compound in a family of naturally occurring organic compounds known as the angular furanocoumarins. Structurally, it can be considered as benzapyra-2-one fused with a furan moiety in the 7,8-position. Angelicin is commonly found in certain Apiaceae and Fabaceae plant species such as Bituminaria bituminosa. It has a skin permeability coefficient (LogKp) of -2.46. The maximum absorption is observed at 300 nm. The 1HNMR spectrum is available; the infrared and mass spectra of angelicin can be found in this database. The sublimation of angelicin occurs at 120 °C and the pressure of 0.13 Pa. Angelicin is a coumarine.
The biosynthesis of phenylpropanoids involves a number of enzymes.
Bacterial leucyl aminopeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Xaa-methyl-His dipeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Penicillopepsin is an enzyme. This enzyme catalyses the following chemical reaction
Beta-lytic metalloendopeptidase is an enzyme. This enzyme catalyses the following chemical reaction

Marmesin (nodakenetin) is a chemical compound precursor in psoralen and linear furanocoumarins biosynthesis.

Paroxypropione, also known as paraoxypropiophenone, is a synthetic nonsteroidal estrogen which has been used medically as an antigonadotropin in Spain and Italy but appears to no longer be marketed. It was first synthesized in 1902. The antigonadotropic properties of the drug were discovered in 1951 and it entered clinical use shortly thereafter.

Hydroxyprogesterone heptanoate (OHPH), also known as hydroxyprogesterone enanthate (OHPE) and sold under the brand names H.O.P., Lutogil A.P., and Lutogyl A.P. among others, is a progestin medication used for progestogenic indications. It has been formulated both alone and in together with estrogens, androgens/anabolic steroids, and other progestogens in several combination preparations. OHPH is given by injection into muscle at regular intervals.

Estradiol sulfate (E2S), or 17β-estradiol 3-sulfate, is a natural, endogenous steroid and an estrogen ester. E2S itself is biologically inactive, but it can be converted by steroid sulfatase into estradiol, which is a potent estrogen. Simultaneously, estrogen sulfotransferases convert estradiol to E2S, resulting in an equilibrium between the two steroids in various tissues. Estrone and E2S are the two immediate metabolic sources of estradiol. E2S can also be metabolized into estrone sulfate (E1S), which in turn can be converted into estrone and estradiol. Circulating concentrations of E2S are much lower than those of E1S. High concentrations of E2S are present in breast tissue, and E2S has been implicated in the biology of breast cancer via serving as an active reservoir of estradiol.












