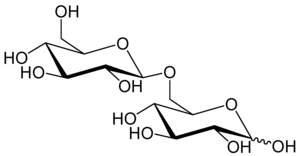
A disaccharide is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, lactose, and maltose.

Cellulase is any of several enzymes produced chiefly by fungi, bacteria, and protozoans that catalyze cellulolysis, the decomposition of cellulose and of some related polysaccharides:

Cellobiose is a disaccharide with the formula (C6H7(OH)4O)2O. It is classified as a reducing sugar - any sugar that possesses the ability or function of a reducing agent. The chemical structure of cellulose is derived from the condensation of a pair of β-glucose molecules forming a β(1→4) bond. It can be hydrolyzed to glucose enzymatically or with acid. Cellobiose has eight free alcohol (OH) groups, one acetal linkage, and one hemiacetal linkage, which give rise to strong inter- and intramolecular hydrogen bonds. It is a white solid.

Cycloamyloses are cyclic α-1,4 linked glucans comprising dozens or hundreds of glucose units. Chemically they are similar to the much smaller cyclodextrins, which are typically composed of 6, 7 or 8 glucose units.

Lichenin, also known as lichenan or moss starch, is a complex glucan occurring in certain species of lichens. It can be extracted from Cetraria islandica. It has been studied since about 1957.
A glucan is a polysaccharide derived from D-glucose, linked by glycosidic bonds. Glucans are noted in two forms: alpha glucans and beta glucans. Many beta-glucans are medically important. They represent a drug target for antifungal medications of the echinocandin class.
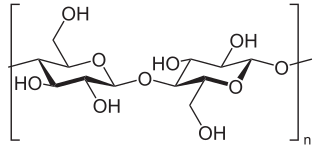
Beta-glucans, β-glucans comprise a group of β-D-glucose polysaccharides (glucans) naturally occurring in the cell walls of cereals, bacteria, and fungi, with significantly differing physicochemical properties dependent on source. Typically, β-glucans form a linear backbone with 1–3 β-glycosidic bonds but vary with respect to molecular mass, solubility, viscosity, branching structure, and gelation properties, causing diverse physiological effects in animals.

Zymosan is a beta-glucan with repeating glucose units connected by β-1,3-glycosidic linkages. It binds to TLR 2 and Dectin-1 (CLEC7A). Zymosan is a ligand found on the surface of fungi, like yeast.

Pleuran is an insoluble polysaccharide, isolated from Pleurotus ostreatus.

α-Glucans (alpha-glucans) are polysaccharides of D-glucose monomers linked with glycosidic bonds of the alpha form. α-Glucans use cofactors in a cofactor site in order to activate a glucan phosphorylase enzyme. This enzyme causes a reaction that transfers a glucosyl portion between orthophosphate and α-I,4-glucan. The position of the cofactors to the active sites on the enzyme are critical to the overall reaction rate thus, any alteration to the cofactor site leads to the disruption of the glucan binding site.

Glucanases are enzymes that break down large polysaccharides via hydrolysis. The product of the hydrolysis reaction is called a glucan, a linear polysaccharide made of up to 1200 glucose monomers, held together with glycosidic bonds. Glucans are abundant in the endosperm cell walls of cereals such as barley, rye, sorghum, rice, and wheat. Glucanases are also referred to as lichenases, hydrolases, glycosidases, glycosyl hydrolases, and/or laminarinases. Many types of glucanases share similar amino acid sequences but vastly different substrates. Of the known endo-glucanases, 1,3-1,4-β-glucanase is considered the most active.
Glucan endo-1,3-β-D-glucosidase is an enzyme with systematic name 3-β-D-glucan glucanohydrolase. This enzyme catalyses the following chemical reaction

Glucan 1,3-β-glucosidase is an enzyme with systematic name 3-β-D-glucan glucohydrolase. It catalyses the successive hydrolysis of β-D-glucose units from the non-reducing ends of (1→3)-β-D-glucans, releasing α-glucose.
Glucan 1,6-α-glucosidase is an enzyme with systematic name glucan 6-α-D-glucohydrolase. It catalyses the hydrolysis of (1→6)-α-D-glucosidic linkages in (1→6)-α-D-glucans and derived oligosaccharides
Lichenase is an enzyme with systematic name (1→3)-(1→4)-β-D-glucan 4-glucanohydrolase. It was named after its activity in on lichenin.
Glucan 1,6-α-isomaltosidase is an enzyme with systematic name 6-α-D-glucan isomaltohydrolase. It catalyses hydrolysis of (1→6)-β-D-glucosidic linkages in polysaccharides, to remove successive isomaltose units from the non-reducing ends of the chains
Mannosyl-oligosaccharide 1,3-1,6-α-mannosidase, also known as Golgi α-mannosidase II, is an enzyme with systematic name (1→3)-(1→6)-mannosyl-oligosaccharide α-D-mannohydrolase. It catalyses the hydrolysis of the terminal (1→3)- and (1→6)-linked α-D-mannose residues in the mannosyl-oligosaccharide Man5(GlcNAc)3.
Penicillium multicolor is an anamorph species of the genus Penicillium which produces alpha-L-fucosidase, tilactase, sclerotiorin, 8-O-Methylsclerotiorinamine, multicolosic acid and isochromophilones.

Oat β-glucans are water-soluble β-glucans derived from the endosperm of oat kernels known for their dietary contribution as components of soluble fiber. Due to their property to lower serum total cholesterol and low-density lipoprotein cholesterol, and potentially reduce the risk of cardiovascular diseases, oat β-glucans have been assigned a qualified health claim by the European Food Safety Authority and the US Food and Drug Administration.

Botryosphaeran is an exopolysaccharide (EPS) produced by the ascomyceteous fungus Botryosphaeria rhodina. Characterization of the chemical structure of botryosphaeran showed this EPS to be a (1→3)(1→6)-β-D-glucan. This particular β-glucan can be produced by several strains of Botryosphaeria rhodina that include: MAMB-05, DABAC-P82, and RCYU 30101. Botryosphaeran exhibits interesting rheological properties and novel biological functions including hypoglycaemia, hypocholesterolaemia, anti-atheroslerosis and anti-cancer activity, with potential commercial applications. Three cosmetic products formulated with botryosphaeran have been developed to promote skin health and treat skin conditions for future intended commercialization purposes.