
A carboxylic acid is an organic acid that contains a carboxyl group (C(=O)OH) attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

An ester is a chemical compound derived from an acid in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.
The cumene process is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene, the intermediate material during the process. It was invented by R. Ūdris and P. Sergeyev in 1942 (USSR)., and independently by Heinrich Hock in 1944
A nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
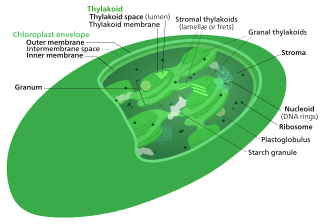
The Calvin cycle,light-independent reactions, bio synthetic phase,dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis are the chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into glucose. The Calvin cycle is present in all photosynthetic eukaryotes and also many photosynthetic bacteria. In plants, these reactions occur in the stroma, the fluid-filled region of a chloroplast outside the thylakoid membranes. These reactions take the products of light-dependent reactions and perform further chemical processes on them. The Calvin cycle uses the chemical energy of ATP and reducing power of NADPH from the light dependent reactions to produce sugars for the plant to use. These substrates are used in a series of reduction-oxidation reactions to produce sugars in a step-wise process; there is no direct reaction that converts several molecules of CO2 to a sugar. There are three phases to the light-independent reactions, collectively called the Calvin cycle: carboxylation, reduction reactions, and ribulose 1,5-bisphosphate (RuBP) regeneration.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2.

Delavirdine (DLV) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) marketed by ViiV Healthcare. It is used as part of highly active antiretroviral therapy (HAART) for the treatment of human immunodeficiency virus (HIV) type 1. It is presented as the mesylate. The recommended dosage is 400 mg, three times a day.
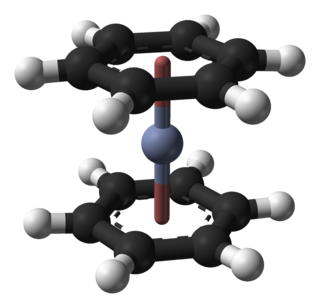
Bis(benzene)chromium is the organometallic compound with the formula Cr(η6-C6H6)2. It is sometimes called dibenzenechromium. The compound played an important role in the development of sandwich compounds in organometallic chemistry and is the prototypical complex containing two arene ligands.
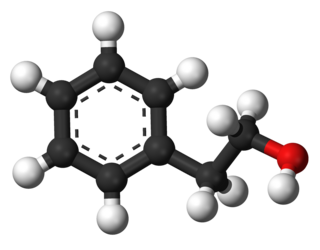
Phenethyl alcohol, or 2-phenylethanol, is the organic compound that consists of a phenethyl group (C6H5CH2CH2) attached to OH. It is a colourless liquid that is slightly soluble in water (2 ml/100 ml H2O), but miscible with most organic solvents. It occurs widely in nature, being found in a variety of essential oils. It has a pleasant floral odor.

Disodium tetracarbonylferrate is the organoiron compound with the formula Na2[Fe(CO)4]. It is always used as a solvate, e.g., with tetrahydrofuran or dimethoxyethane, which bind to the sodium cation. An oxygen-sensitive colourless solid, it is a reagent in organometallic and organic chemical research. The dioxane solvated sodium salt is known as Collman's reagent, in recognition of James P. Collman, an early popularizer of its use.

Oxazoline is a five-membered heterocyclic chemical compound containing one atom each of oxygen and nitrogen. It was likely first synthesized in 1884 but it was not until 5 years later that Siegmund Gabriel correctly assigned the structure. It was named in-line with the Hantzsch–Widman nomenclature and is part of a family of heterocyclic compounds, where it exists between oxazole and oxazolidine in terms of saturation.

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field.
The [2,3]-Wittig rearrangement is the transformation of an allylic ether into a homoallylic alcohol via a concerted, pericyclic process. Because the reaction is concerted, it exhibits a high degree of stereocontrol, and can be employed early in a synthetic route to establish stereochemistry. The Wittig rearrangement requires strongly basic conditions, however, as a carbanion intermediate is essential. [1,2]-Wittig rearrangement is a competitive process.

In organic chemistry, carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent.
Reductions with metal alkoxyaluminium hydrides are chemical reactions that involve either the net hydrogenation of an unsaturated compound or the replacement of a reducible functional group with hydrogen by metal alkoxyaluminium hydride reagents.
The molecular formula C22H42O4 (molar mass: 370.56 g/mol) may refer to:

Chloro(pyridine)cobaloxime is a coordination compound containing a CoIII center with octahedral coordination. It has been considered as a model compound of vitamin B12 for studying the properties and mechanism of action of the vitamin. It belongs to a class of bis(dimethylglyoximato)cobalt(III) complexes with different axial ligands, called cobaloximes. Chloro(pyridine)cobaloxime is a yellow-brown powder that is sparingly soluble in most solvents, including water.
Borane–tetrahydrofuran is a dipolar bond charge-transfer complex composed of borane and tetrahydrofuran (THF). These solutions are used for reductions and hydroboration, reactions that are useful in synthesis of organic compounds.
Iron(II) hydride, systematically named iron dihydride and poly(dihydridoiron) is solid inorganic compound with the chemical formula (FeH
2)
n. ). It is kinetically unstable at ambient temperature, and as such, little is known about its bulk properties. However, it known as a black, amorphous powder, which was synthesised for the first time in 2014.












