The Thermoproteota are prokaryotes that have been classified as a phylum of the domain Archaea. Initially, the Thermoproteota were thought to be sulfur-dependent extremophiles but recent studies have identified characteristic Thermoproteota environmental rRNA indicating the organisms may be the most abundant archaea in the marine environment. Originally, they were separated from the other archaea based on rRNA sequences; other physiological features, such as lack of histones, have supported this division, although some crenarchaea were found to have histones. Until 2005 all cultured Thermoproteota had been thermophilic or hyperthermophilic organisms, some of which have the ability to grow at up to 113 °C. These organisms stain Gram negative and are morphologically diverse, having rod, cocci, filamentous and oddly-shaped cells. Recent evidence shows that some members of the Thermoproteota are methanogens.

The Korarchaeota is a proposed phylum within the Archaea. The name is derived from the Greek noun koros or kore, meaning young man or young woman, and the Greek adjective archaios which means ancient. They are also known as Xenarchaeota. The name is equivalent to Candidatus Korarchaeota, and they go by the name Xenarchaeota or Xenarchaea as well.
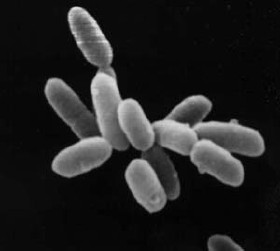
Euryarchaeota is a kingdom of archaea. Euryarchaeota are highly diverse and include methanogens, which produce methane and are often found in intestines; halobacteria, which survive extreme concentrations of salt; and some extremely thermophilic aerobes and anaerobes, which generally live at temperatures between 41 and 122 °C. They are separated from the other archaeans based mainly on rRNA sequences and their unique DNA polymerase. The only validly published name for this group under the Prokaryotic Code is Methanobacteriati.
The Aquificota phylum is a diverse collection of bacteria that live in harsh environmental settings. The name Aquificota was given to this phylum based on an early genus identified within this group, Aquifex, which is able to produce water by oxidizing hydrogen. They have been found in springs, pools, and oceans. They are autotrophs, and are the primary carbon fixers in their environments. These bacteria are Gram-negative, non-spore-forming rods. They are true bacteria as opposed to the other inhabitants of extreme environments, the Archaea.
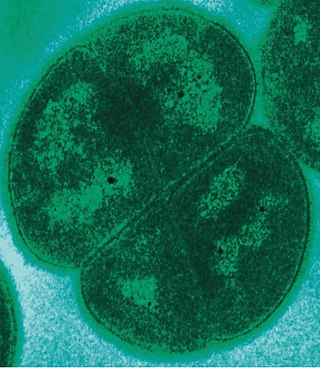
Deinococcota is a phylum of bacteria with a single class, Deinococci, that are highly resistant to environmental hazards, also known as extremophiles. These bacteria have thick cell walls that give them gram-positive stains, but they include a second membrane and so are closer in structure to those of gram-negative bacteria.
The Thermomicrobia is a group of thermophilic green non-sulfur bacteria. Based on species Thermomicrobium roseum and Sphaerobacter thermophilus, this bacteria class has the following description:
Chrysiogenaceae is a family of bacteria.
The Thermoprotei is a class of the Thermoproteota.
Mollicutes is a class of bacteria distinguished by the absence of a cell wall. The word "Mollicutes" is derived from the Latin mollis, and cutis. Individuals are very small, typically only 0.2–0.3 μm in size and have a very small genome size. They vary in form, although most have sterols that make the cell membrane somewhat more rigid. Many are able to move about through gliding, but members of the genus Spiroplasma are helical and move by twisting. The best-known genus in the Mollicutes is Mycoplasma. Colonies show the typical "fried-egg" appearance.
Archaeoglobaceae are a family of the Archaeoglobales. All known genera within the Archaeoglobaceae are hyperthermophilic and can be found near undersea hydrothermal vents. Archaeoglobaceae are the only family in the order Archaeoglobales, which is the only order in the class Archaeoglobi.
The Thermotogota are a phylum of the domain Bacteria. The phylum contains a single class, Thermotogae. The phylum Thermotogota is composed of Gram-negative staining, anaerobic, and mostly thermophilic and hyperthermophilic bacteria. It's the sole phylum in the kingdom Thermotogati.

Sulfur-reducing bacteria are microorganisms able to reduce elemental sulfur (S0) to hydrogen sulfide (H2S). These microbes use inorganic sulfur compounds as electron acceptors to sustain several activities such as respiration, conserving energy and growth, in absence of oxygen. The final product of these processes, sulfide, has a considerable influence on the chemistry of the environment and, in addition, is used as electron donor for a large variety of microbial metabolisms. Several types of bacteria and many non-methanogenic archaea can reduce sulfur. Microbial sulfur reduction was already shown in early studies, which highlighted the first proof of S0 reduction in a vibrioid bacterium from mud, with sulfur as electron acceptor and H
2 as electron donor. The first pure cultured species of sulfur-reducing bacteria, Desulfuromonas acetoxidans, was discovered in 1976 and described by Pfennig Norbert and Biebel Hanno as an anaerobic sulfur-reducing and acetate-oxidizing bacterium, not able to reduce sulfate. Only few taxa are true sulfur-reducing bacteria, using sulfur reduction as the only or main catabolic reaction. Normally, they couple this reaction with the oxidation of acetate, succinate or other organic compounds. In general, sulfate-reducing bacteria are able to use both sulfate and elemental sulfur as electron acceptors. Thanks to its abundancy and thermodynamic stability, sulfate is the most studied electron acceptor for anaerobic respiration that involves sulfur compounds. Elemental sulfur, however, is very abundant and important, especially in deep-sea hydrothermal vents, hot springs and other extreme environments, making its isolation more difficult. Some bacteria – such as Proteus, Campylobacter, Pseudomonas and Salmonella – have the ability to reduce sulfur, but can also use oxygen and other terminal electron acceptors.

In taxonomy, the Thermoplasmata are a class of the Euryarchaeota.

In the taxonomy of microorganisms, the Methanomicrobia are a class of the Euryarchaeota.

Methanococci is a class of methanogenic archaea in the phylum Euryarchaeota. They can be mesophilic, thermophilic or hyperthermophilic.

The Desulfurococcales is an order of the Thermoprotei, part of the kingdom Archaea. The order encompasses some genera which are all thermophilic, autotrophs which utilise chemical energy, typically by reducing sulfur compounds using hydrogen. Desulfurococcales cells are either regular or irregular coccus in shape, with forms of either discs or dishes. These cells can be single, in pairs, in short chains, or in aciniform formation.
Methanocaldococcus formerly known as Methanococcus is a genus of coccoid methanogen archaea. They are all mesophiles, except the thermophilic M. thermolithotrophicus and the hyperthermophilic M. jannaschii. The latter was discovered at the base of a “white smoker” chimney at 21°N on the East Pacific Rise and it was the first archaean genome to be completely sequenced, revealing many novel and eukaryote-like elements.

Thermotoga maritima is a hyperthermophilic, anaerobic organism that is a member of the order Thermotogales. T. maritima is well known for its ability to produce hydrogen (clean energy) and it is the only fermentative bacterium that has been shown to produce hydrogen more than the Thauer limit (>4 mol H2 /mol glucose). It employs [FeFe]-hydrogenases to produce hydrogen gas (H2) by fermenting many different types of carbohydrates.
Thermotoga naphthophila is a hyperthermophilic, anaerobic, non-spore-forming, rod-shaped fermentative heterotroph, with type strain RKU-10T.

The evolution of bacteria has progressed over billions of years since the Precambrian time with their first major divergence from the archaeal/eukaryotic lineage roughly 3.2-3.5 billion years ago. This was discovered through gene sequencing of bacterial nucleoids to reconstruct their phylogeny. Furthermore, evidence of permineralized microfossils of early prokaryotes was also discovered in the Australian Apex Chert rocks, dating back roughly 3.5 billion years ago during the time period known as the Precambrian time. This suggests that an organism in of the phylum Thermotogota was the most recent common ancestor of modern bacteria.