
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
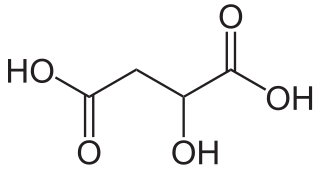
Malic acid is an organic compound with the molecular formula C4H6O5. It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms, though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle.

In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.

In organic chemistry, a dicarbonyl is a molecule containing two carbonyl groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.
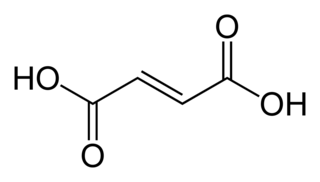
Fumaric acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297. The salts and esters are known as fumarates. Fumarate can also refer to the C
4H
2O2−
4 ion (in solution). Fumaric acid is the trans isomer of butenedioic acid, while maleic acid is the cis isomer.
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes, alkynes, or azo compounds are cleaved with ozone. Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl group while azo compounds form nitrosamines. The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions.

An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word acid in the name of the parent carboxylic acid by the word anhydride. Thus, (CH3CO)2O is called acetic anhydride.Mixed (or unsymmetrical) acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride").

Maleic acid or cis-butenedioic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCH=CHCO2H. Maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer. It is mainly used as a precursor to fumaric acid, and relative to its parent maleic anhydride, maleic acid has few applications.

Maleic anhydride is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.

Itaconic acid, or methylidenesuccinic acid, is an organic compound. This dicarboxylic acid is a white solid that is soluble in water, ethanol, and acetone. Historically, itaconic acid was obtained by the distillation of citric acid, but currently it is produced by fermentation. The name itaconic acid was devised as an anagram of aconitic acid, another derivative of citric acid.

Methacrylic acid, abbreviated MAA, is an organic compound. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).

Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for esterification. It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents.

Succinic anhydride, is an organic compound with the molecular formula (CH2CO)2O. This colorless solid is the acid anhydride of succinic acid.

Anthranilic acid is an aromatic acid with the formula C6H4(NH2)(CO2H) and has a sweetish taste. The molecule consists of a benzene ring, ortho-substituted with a carboxylic acid and an amine. As a result of containing both acidic and basic functional groups, the compound is amphoteric. Anthranilic acid is a white solid when pure, although commercial samples may appear yellow. The anion [C6H4(NH2)(CO2)]−, obtained by the deprotonation of anthranilic acid, is called anthranilate. Anthranilic acid was once thought to be a vitamin and was referred to as vitamin L1 in that context, but it is now known to be non-essential in human nutrition.
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbonylation also refers to oxidation of protein side chains.

Alkenyl succinic anhydrides (ASA) are derivatives of succinic anhydrides. One H of the succinic anhydride ring is replaced with an iso-alkenyl chain (C14 to C22). ASA's are colorless and usually viscous liquids. They are widely used, especially in surface sizing of paper, paperboard, and cardboard, as well as in the hydrophobicization of cellulose fibers. Products treated with it show reduced penetration of aqueous media, such as inks or drinks (like milk or fruit juices).
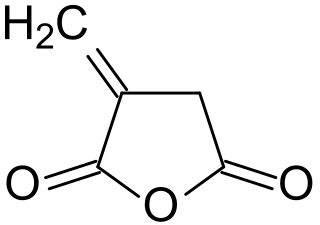
Itaconic anhydride is the cyclic anhydride of itaconic acid and is obtained by the pyrolysis of citric acid. It is a colourless, crystalline solid, which dissolves in many polar organic solvents and hydrolyzes forming itaconic acid. Itaconic anhydride and its derivative itaconic acid have been promoted as biobased "platform chemicals" and bio- building blocks.) These expectations, however, have not been fulfilled.

α,β-Unsaturated carbonyl compounds are organic compounds with the general structure (O=CR)−Cα=Cβ-R. Such compounds include enones and enals. In these compounds the carbonyl group is conjugated with an alkene. Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β-carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction.



















