A monomer is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.

Vulcanization is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to include the hardening of other (synthetic) rubbers via various means. Examples include silicone rubber via room temperature vulcanizing and chloroprene rubber (neoprene) using metal oxides.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

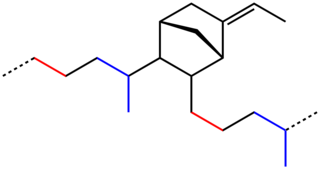
1,3-Butadiene is the organic compound with the formula CH2=CH-CH=CH2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene.

Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene. These materials have good abrasion resistance and good aging stability when protected by additives. In 2012, more than 5.4 million tonnes of SBR were processed worldwide. About 50% of car tires are made from various types of SBR. The styrene/butadiene ratio influences the properties of the polymer: with high styrene content, the rubbers are harder and less rubbery. SBR is not to be confused with the thermoplastic elastomer, styrene-butadiene block copolymer, although being derived from the same monomers.
An elastomer is a polymer with viscoelasticity and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a portmanteau of elastic polymer, is often used interchangeably with rubber, although the latter is preferred when referring to vulcanisates. Each of the monomers which link to form the polymer is usually a compound of several elements among carbon, hydrogen, oxygen and silicon. Elastomers are amorphous polymers maintained above their glass transition temperature, so that considerable molecular reconformation is feasible without breaking of covalent bonds. At ambient temperatures, such rubbers are thus relatively compliant and deformable.

An O-ring, also known as a packing or a toric joint, is a mechanical gasket in the shape of a torus; it is a loop of elastomer with a round cross-section, designed to be seated in a groove and compressed during assembly between two or more parts, forming a seal at the interface.
EPDM rubber is a type of synthetic rubber that is used in many applications.

Butyl rubber, sometimes just called "butyl", is a synthetic rubber, a copolymer of isobutylene with isoprene. The abbreviation IIR stands for isobutylene isoprene rubber. Polyisobutylene, also known as "PIB" or polyisobutene, (C4H8)n, is the homopolymer of isobutylene, or 2-methyl-1-propene, on which butyl rubber is based. Butyl rubber is produced by polymerization of about 98% of isobutylene with about 2% of isoprene. Structurally, polyisobutylene resembles polypropylene, but has two methyl groups substituted on every other carbon atom, rather than one. Polyisobutylene is a colorless to light yellow viscoelastic material. It is generally odorless and tasteless, though it may exhibit a slight characteristic odor.

Polybutadiene [butadiene rubber, BR] is a synthetic rubber. It offers high elasticity, high resistance to wear, good strength even without fillers, and excellent abrasion resistance when filled and vulcanized. "Polybutadiene" is a collective name for homopolymers formed from the polymerization of the monomer 1,3-butadiene. The IUPAC refers to polybutadiene as "poly(buta-1,3-diene)". Historically, an early generation of synthetic polybutadiene rubber produced in Germany by Bayer using sodium as a catalyst was known as "Buna rubber". Polybutadiene is typically crosslinked with sulphur, however, it has also been shown that it can be UV cured when bis-benzophenone additives are incorporated into the formulation.
Nitrile rubber, also known as nitrile butadiene rubber, NBR, Buna-N, and acrylonitrile butadiene rubber, is a synthetic rubber derived from acrylonitrile (ACN) and butadiene. Trade names include Perbunan, Nipol, Krynac and Europrene. This rubber is unusual in being resistant to oil, fuel, and other chemicals.
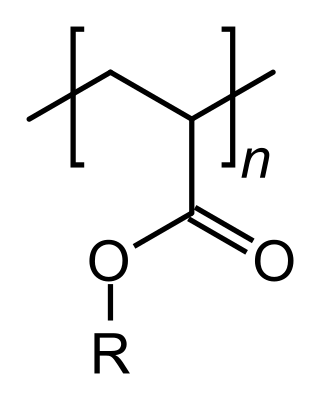
An acrylate polymer is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity.
Solution polymerization is a method of industrial polymerization. In this procedure, a monomer is dissolved in a non-reactive solvent that contains a catalyst or initiator.

Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking. The problem was formerly very common, especially in tires, but is now rarely seen in those products owing to preventive measures.
In polymer chemistry, a comonomer refers to a polymerizable precursor to a copolymer aside from the principal monomer. In some cases, only small amounts of a comonomer are employed, in other cases substantial amounts of comonomers are used. Furthermore, in some cases, the comonomers are statistically incorporated within the polymer chain, whereas in other cases, they aggregate. The distribution of comonomers is referred to as the "blockiness" of a copolymer.

An antiozonant, also known as anti-ozonant, is an organic compound that prevents or retards damage caused by ozone. The most important antiozonants are those which prevent degradation of elastomers like rubber. A number of research projects study the application of another type of antiozonants to protect plants as well as salmonids that are affected by the chemicals.

The Charles Goodyear Medal is the highest honor conferred by the American Chemical Society, Rubber Division. Established in 1941, the award is named after Charles Goodyear, the discoverer of vulcanization, and consists of a gold medal, a framed certificate and prize money. The medal honors individuals for "outstanding invention, innovation, or development which has resulted in a significant change or contribution to the nature of the rubber industry". Awardees give a lecture at an ACS Rubber Division meeting, and publish a review of their work in the society's scientific journal Rubber Chemistry and Technology.

Acrylonitrile styrene acrylate (ASA), also called acrylic styrene acrylonitrile, is an amorphous thermoplastic developed as an alternative to acrylonitrile butadiene styrene (ABS), that has improved weather resistance. It is an acrylate rubber-modified styrene acrylonitrile copolymer. It is used for general prototyping in 3D printing, where its UV resistance and mechanical properties make it an excellent material for use in fused filament fabrication printers, particularly for outdoor applications. ASA is also widely used in the automotive industry.
Walter Bock was a German chemist who developed styrene-butadiene copolymer by emulsion polymerization as a synthetic rubber (SBR).

Poly(butyl acrylate) (PBA) is a family of organic polymers with the formula (CH2CHCO2CH2CH2CH2CH3)n. It is a synthetic acrylate polymer derived from butyl acrylate monomer. The polymers are colorless. This homopolymer is far less important than copolymers derived from methyl acrylate and other monomers. It has a low glass-transition temperature of about -43 °C (20 °C).

















