
Kevlar (para-aramid) is a strong, heat-resistant synthetic fiber, related to other aramids such as Nomex and Technora. Developed by Stephanie Kwolek at DuPont in 1965, the high-strength material was first used commercially in the early 1970s as a replacement for steel in racing tires. It is typically spun into ropes or fabric sheets that can be used as such, or as an ingredient in composite material components.

Vulcanization is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to include the hardening of other (synthetic) rubbers via various means. Examples include silicone rubber via room temperature vulcanizing and chloroprene rubber (neoprene) using metal oxides.
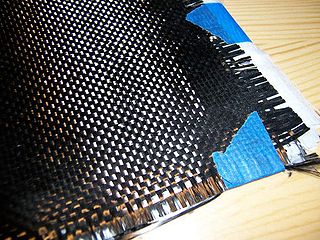
Carbon fibers or carbon fibres are fibers about 5 to 10 micrometers (0.00020–0.00039 in) in diameter and composed mostly of carbon atoms. Carbon fibers have several advantages: high stiffness, high tensile strength, high strength to weight ratio, high chemical resistance, high-temperature tolerance, and low thermal expansion. These properties have made carbon fiber very popular in aerospace, civil engineering, military, motorsports, and other competition sports. However, they are relatively expensive compared to similar fibers, such as glass fiber, basalt fibers, or plastic fibers.

Neoprene is a family of synthetic rubbers that are produced by polymerization of chloroprene. Neoprene exhibits good chemical stability and maintains flexibility over a wide temperature range. Neoprene is sold either as solid rubber or in latex form and is used in a wide variety of commercial applications, such as laptop sleeves, orthopaedic braces, electrical insulation, medical gloves, liquid and sheet-applied elastomeric membranes or flashings, and automotive fan belts.

Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene. These materials have good abrasion resistance and good aging stability when protected by additives. In 2012, more than 5.4 million tonnes of SBR were processed worldwide. About 50% of car tires are made from various types of SBR. The styrene/butadiene ratio influences the properties of the polymer: with high styrene content, the rubbers are harder and less rubbery. SBR is not to be confused with the thermoplastic elastomer, styrene-butadiene block copolymer, although being derived from the same monomers.

An O-ring, also known as a packing or a toric joint, is a mechanical gasket in the shape of a torus; it is a loop of elastomer with a round cross-section, designed to be seated in a groove and compressed during assembly between two or more parts, forming a seal at the interface.
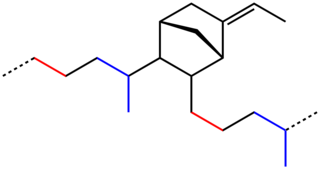
EPDM rubber is a type of synthetic rubber that is used in many applications.
A synthetic rubber is an artificial elastomer. They are polymers synthesized from petroleum byproducts. About 32 million metric tons of rubbers are produced annually in the United States, and of that amount two thirds are synthetic. Synthetic rubber, just like natural rubber, has many uses in the automotive industry for tires, door and window profiles, seals such as O-rings and gaskets, hoses, belts, matting, and flooring. They offer a different range of physical and chemical properties which can improve the reliability of a given product or application. Synthetic rubbers are superior to natural rubbers in two major respects: thermal stability, and resistance to oils and related compounds. They are more resistant to oxidizing agents, such as oxygen and ozone which can reduce the life of products like tires.

Polybutadiene [butadiene rubber, BR] is a synthetic rubber. It offers high elasticity, high resistance to wear, good strength even without fillers, and excellent abrasion resistance when filled and vulcanized. "Polybutadiene" is a collective name for homopolymers formed from the polymerization of the monomer 1,3-butadiene. The IUPAC refers to polybutadiene as "poly(buta-1,3-diene)". Historically, an early generation of synthetic polybutadiene rubber produced in Germany by Bayer using sodium as a catalyst was known as "Buna rubber". Polybutadiene is typically crosslinked with sulphur, however, it has also been shown that it can be UV cured when bis-benzophenone additives are incorporated into the formulation.
Nitrile rubber, also known as nitrile butadiene rubber, NBR, Buna-N, and acrylonitrile butadiene rubber, is a synthetic rubber derived from acrylonitrile (ACN) and butadiene. Trade names include Perbunan, Nipol, Krynac and Europrene. This rubber is unusual in being resistant to oil, fuel, and other chemicals.

Zytel is a trademark owned by Celanese and used to make different high-strength, abrasion, and impact-resistant thermoplastic polyamide formulations, in the family of material more commonly known as nylon. The Zytel product line is based mostly on nylon 66, but also includes grades based on nylon 6 as a matrix, long chain nylons such as nylon 610, and copolymers including a transparent resin called Zytel 330. Resins based on polyphthalamides are branded 'Zytel HTN'. The Zytel product range takes advantage of the fact that nylons are one of the most compatible polymers with modifiers and so offers grades with varying degrees of fiberglass, from 13% to 60%, rubber toughened resins and flame retarded grades. Nylon resins with mineral reinforcements are branded 'Minlon'.

Silicone rubber is an elastomer composed of silicone—itself a polymer—containing silicon together with carbon, hydrogen, and oxygen. Silicone rubbers are widely used in industry, and there are multiple formulations. Silicone rubbers are often one- or two-part polymers, and may contain fillers to improve properties or reduce cost. Silicone rubber is generally non-reactive, stable, and resistant to extreme environments and temperatures from −55 to 300 °C while still maintaining its useful properties. Due to these properties and its ease of manufacturing and shaping, silicone rubber can be found in a wide variety of products, including voltage line insulators; automotive applications; cooking, baking, and food storage products; apparel such as undergarments, sportswear, and footwear; electronics; medical devices and implants; and in home repair and hardware, in products such as silicone sealants.

Pneumatic tires are manufactured according to relatively standardized processes and machinery, in around 455 tire factories in the world. With over 1 billion tires manufactured worldwide annually, the tire industry is a major consumer of natural rubber. Tire factories start with bulk raw materials such as synthetic rubber, carbon black, and chemicals and produce numerous specialized components that are assembled and cured.
Thermoplastic vulcanizates (TPV) are dynamically vulcanized alloys consisting mostly of fully cured EPDM rubber particles encapsulated in a polypropylene (PP) matrix. They are part of the thermoplastic elastomer (TPE) family of polymers but are closest in elastomeric properties to EPDM thermoset rubber, combining the characteristics of vulcanized rubber with the processing properties of thermoplastics. There are almost 100 grades in the S portfolio that are used globally in the automotive, household appliance, electrical, construction, and healthcare markets. The name Santoprene was trademarked in 1977 by Monsanto, and the trademark is now owned by Celanese. Similar material is available from Elastron and others.
Novel polymeric alloy (NPA) is a polymeric alloy composed of polyolefin and thermoplastic engineering polymer with enhanced engineering properties. NPA was developed for use in geosynthetics. One of the first commercial NPA applications was in the manufacturer of polymeric strips used to form Neoloy® cellular confinement systems (geocells).

A metal hose is a flexible metal line element. There are two basic types of metal hose that differ in their design and application: stripwound hoses and corrugated hoses.
David Spence was one of the pioneering rubber chemists. He helped the war effort during the Second World War by devising new ways of extracting natural rubbers from plants, and worked to improve the processing of the rubber. Over the course of his career, he worked to improve the dyeing processes for rubber products and the vulcanization of rubber, and in developing new accelerants for strengthening lower-quality natural rubber. In 1941, he became the first recipient of the Charles Goodyear Medal, awarded by the American Chemical Society.

Kumho Petrochemical Co., Ltd. is a multinational chemical company based in South Korea, with headquarters in Seoul. It was founded in 1970 when Kumho Group struggled to secure raw materials for its bus and tire businesses. Kumho Petrochemical has a global market leadership in the manufacturing of synthetic rubbers with the world's largest production capacity based on SBR and BR by IISRP 2012. It focuses on synthetic rubbers, synthetic resins, specialty chemicals, electronic chemicals, energy, building materials and advanced materials as its core business.
Walter Bock was a German chemist who developed styrene-butadiene copolymer by emulsion polymerization as a synthetic rubber (SBR).

Sulfur vulcanization is a chemical process for converting natural rubber or related polymers into materials of varying hardness, elasticity, and mechanical durability by heating them with sulfur or sulfur-containing compounds. Sulfur forms cross-linking bridges between sections of polymer chains which affects the mechanical and electronic properties. Many products are made with vulcanized rubber, including tires, shoe soles, hoses, and conveyor belts. The term vulcanization is derived from Vulcan, the Roman god of fire.











