A β-lactam (beta-lactam) ring is a four-membered lactam. A lactam is a cyclic amide, and beta-lactams are named so because the nitrogen atom is attached to the β-carbon atom relative to the carbonyl. The simplest β-lactam possible is 2-azetidinone. β-lactams are significant structural units of medicines as manifested in many β-lactam antibiotics. Up to 1970, most β-lactam research was concerned with the penicillin and cephalosporin groups, but since then, a wide variety of structures have been described.

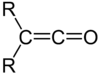
In organic chemistry, a ketene is an organic compound of the form RR'C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refer to the specific compound ethenone H2C=C=O, the simplest ketene.

A polymer is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.

A macromolecule is a very large molecule important to biological processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The most common macromolecules in biochemistry are biopolymers and large non-polymeric molecules such as lipids, nanogels and macrocycles. Synthetic fibers and experimental materials such as carbon nanotubes are also examples of macromolecules.

Leopold Ružička was a Croatian-Swiss scientist and joint winner of the 1939 Nobel Prize in Chemistry "for his work on polymethylenes and higher terpenes" "including the first chemical synthesis of male sex hormones." He worked most of his life in Switzerland, and received eight doctorates honoris causa in science, medicine, and law; seven prizes and medals; and twenty-four honorary memberships in chemical, biochemical, and other scientific societies.

Soft matter or soft condensed matter is a type of matter that can be deformed or structurally altered by thermal or mechanical stress which is of similar magnitude to thermal fluctuations.
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules. However, polymer chemistry is typically related to synthetic and organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, such as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.

Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.
The Passerini reaction is a chemical reaction involving an isocyanide, an aldehyde, and a carboxylic acid to form a α-acyloxy amide. This addition reaction is one of the oldest isocyanide-based multicomponent reactions and was first described in 1921 by Mario Passerini in Florence, Italy. It is typically carried out in aprotic solvents but can also be performed in ionic liquids such as water or deep eutectic solvents. It is a third order reaction; first order in each of the reactants. The Passerini reaction is often used in combinatorial and medicinal chemistry with recent utility in green chemistry and polymer chemistry. As isocyanides exhibit high functional group tolerance, chemoselectivity, regioselectivity, and stereoselectivity, the Passerini reaction has a wide range of synthetic applications.

Polymer science or macromolecular science is a subfield of materials science concerned with polymers, primarily synthetic polymers such as plastics and elastomers. The field of polymer science includes researchers in multiple disciplines including chemistry, physics, and engineering.

Yves Chauvin was a French chemist and Nobel Prize laureate. He was honorary research director at the Institut français du pétrole and a member of the French Academy of Science. He was known for his work for deciphering the process of olefin metathesis for which he was awarded the 2005 Nobel Prize in Chemistry along with Robert H. Grubbs and Richard R. Schrock.
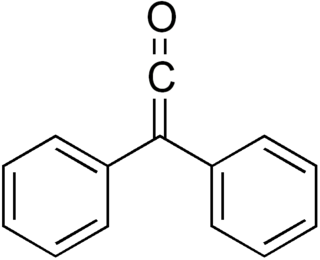
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most stable disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl ketene is a heterocumulene. The most important reaction of diphenyl ketene is the [2+2] cycloaddition at C-C, C-N, C-O, and C-S multiple bonds.

Albert Jakob Eschenmoser (5 August 1925 – 14 July 2023) was a Swiss organic chemist, best known for his work on the synthesis of complex heterocyclic natural compounds, most notably vitamin B12. In addition to his significant contributions to the field of organic synthesis, Eschenmoser pioneered work in the Origins of Life (OoL) field with work on the synthetic pathways of artificial nucleic acids. Before retiring in 2009, Eschenmoser held tenured teaching positions at the ETH Zurich and The Skaggs Institute for Chemical Biology at The Scripps Research Institute in La Jolla, California as well as visiting professorships at the University of Chicago, Cambridge University, and Harvard.

Ethenone is the formal name for ketene, an organic compound with formula C2H2O or H2C=C=O. It is the simplest member of the ketene class. It is an important reagent for acetylations.
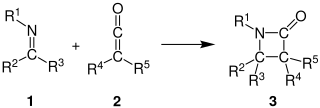
The Staudinger synthesis, also called the Staudinger ketene-imine cycloaddition, is a chemical synthesis in which an imine 1 reacts with a ketene 2 through a non-photochemical 2+2 cycloaddition to produce a β-lactam3. The reaction carries particular importance in the synthesis of β-lactam antibiotics. The Staudinger synthesis should not be confused with the Staudinger reaction, a phosphine or phosphite reaction used to reduce azides to amines.

Automated synthesis or automatic synthesis is a set of techniques that use robotic equipment to perform chemical synthesis in an automated way. Automating processes allows for higher efficiency and product quality although automation technology can be cost-prohibitive and there are concerns regarding overdependence and job displacement. Chemical processes were automated throughout the 19th and 20th centuries, with major developments happening in the previous thirty years, as technology advanced. Tasks that are performed may include: synthesis in variety of different conditions, sample preparation, purification, and extractions. Applications of automated synthesis are found on research and industrial scales in a wide variety of fields including polymers, personal care, and radiosynthesis.

Magda Staudinger was a Latvian biologist and botanist who studied macromolecules with her husband Hermann Staudinger and their application to biology. She was acknowledged as his collaborator when he won the Nobel Prize for Chemistry, and she published seven volumes of his works after his death. She was awarded the Grand Order of the Latvian Academy of Sciences Medal for her contributions to the furtherance of science.
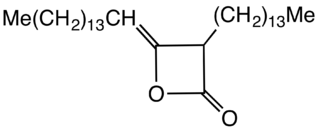
Alkyl ketene dimers (AKDs) are a family of organic compounds based on the 4-membered ring system of oxetan-2-one, which is also the central structural element of propiolactone and diketene. Attached to the oxetane ring of technically relevant alkyl ketene dimers there is a C12 – C16 alkyl group in the 3-position and a C13 – C17 alkylidene group in the 4-position.

Klaus Müllen is a German chemist working in the fields of polymer chemistry, supramolecular chemistry and nanotechnology. He is known for the synthesis and exploration of the properties of graphene-like nanostructures and their potential applications in organic electronics.
The Hermann Staudinger Prize is awarded by the German Chemical Society for groundbreaking work in the field of macromolecular chemistry and polymer science. It comes with a gold medal and a sum of money. It is awarded in even-numbered years and is named after the Nobel Prize in chemistry winner Hermann Staudinger, who is the founder of the field. The prize started in 1970 through donation from BASF and the first prize was handed out in 1971.