An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates.

In chemistry, the term phosphonium describes polyatomic cations with the chemical formula PR+
4. These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.
The Staudinger reaction is a chemical reaction of an organic azide with a phosphine or phosphite produces an iminophosphorane. The reaction was discovered by and named after Hermann Staudinger. The reaction follows this stoichiometry:
The Barton–Kellogg reaction is a coupling reaction between a diazo compound and a thioketone, giving an alkene by way of an episulfide intermediate. The Barton–Kellogg reaction is also known as Barton–Kellogg olefination and Barton olefin synthesis.
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.
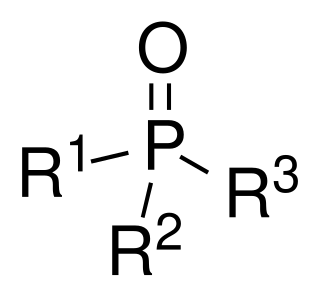
Phosphine oxides are phosphorus compounds with the formula OPX3. When X = alkyl or aryl, these are organophosphine oxides. Triphenylphosphine oxide is an example. An inorganic phosphine oxide is phosphoryl chloride (POCl3). The parent phosphine oxide (H3PO) remains rare and obscure.

Chloro(triphenylphosphine)gold(I) or triphenylphosphinegold(I) chloride is a coordination complex with the formula (Ph3P)AuCl. This colorless solid is a common reagent for research on gold compounds.

Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = C6H5). It is one of the more common phosphine oxides. This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallizing of chemical compounds.
Organophosphines are organophosphorus compounds with the formula PRnH3−n, where R is an organic substituent. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3).

Bis(triphenylphosphine)iminium chloride is the chemical compound with the formula [( 3P)2N]Cl, often abbreviated [(Ph3P)2N]Cl, where Ph is phenyl C6H5, or even abbreviated [PPN]Cl or [PNP]Cl or PPNCl or PNPCl, where PPN or PNP stands for (Ph3P)2N. This colorless salt is a source of the [(Ph3P)2N]+ cation, which is used as an unreactive and weakly coordinating cation to isolate reactive anions. [(Ph3P)2N]+ is a phosphazene.

Diphosphane, or diphosphine, is an inorganic compound with the chemical formula P2H4. This colourless liquid is one of several binary phosphorus hydrides. It is the impurity that typically causes samples of phosphine to ignite in air.
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes, between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.

A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).
The Kirsanov reaction is a method for the synthesis of certain organophosphorus compounds. In this reaction a tertiary phosphine is combined with a halogen and then an amine to give the iminophosphines, which are useful ligands and useful reagents. A typical reaction involves triphenylphosphine with bromine to give bromotriphenylphosphonium bromide:
Methylenetriphenylphosphorane is an organophosphorus compound with the formula Ph3PCH2. It is the parent member of the phosphorus ylides, popularly known as Wittig reagents. It is a highly polar, highly basic species.
In organic chemistry, Wittig reagents are organophosphorus compounds of the formula R3P=CHR', where R is usually phenyl. They are used to convert ketones and aldehydes to alkenes:
The Blum–Ittah aziridine synthesis, also known as the Blum–Ittah-Shahak aziridine synthesis or simply the Blum aziridine synthesis is a name reaction of organic chemistry, for the generation of aziridines from oxiranes.
An organic azide is an organic compound that contains an azide functional group. Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Low molecular weight azides are considered especially hazardous and are avoided. In the research laboratory, azides are precursors to amines. They are also popular for their participation in the "click reaction" between an azide and an alkyne and in Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry.
The Aza-Wittig reaction or is a chemical reaction of a carbonyl group with an aza-ylide, also known as an iminophosphorane. Aza-Wittig reactions are most commonly used to convert aldehydes and ketones to the corresponding imines. The conversion has also been practiced in an intramolecular sense, which is commonly used in the synthesis of N-heterocyclic compounds.











