
In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes and are described by the general structural formula R2C=N+=N−. The simplest example of a diazo compound is diazomethane, CH2N2. Diazo compounds should not be confused with azo compounds or with diazonium compounds.

In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most often, the Wittig reaction is used to introduce a methylene group using methylenetriphenylphosphorane (Ph3P=CH2). Using this reagent, even a sterically hindered ketone such as camphor can be converted to its methylene derivative.
Reductive amination is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is a common method to make amines and is widely used in green chemistry since it can be done catalytically in one-pot under mild conditions. In biochemistry, dehydrogenase enzymes use reductive amination to produce the amino acid, glutamate. Additionally, there is ongoing research on alternative synthesis mechanisms with various metal catalysts which allow the reaction to be less energy taxing, and require milder reaction conditions. Investigation into biocatalysts, such as imine reductases, have allowed for higher selectivity in the reduction of chiral amines which is an important factor in pharmaceutical synthesis.

The Aza-Diels–Alder reaction is a modification of the Diels–Alder reaction wherein a nitrogen replaces sp2 carbon. The nitrogen atom can be part of the diene or the dienophile.
A pinacol coupling reaction is an organic reaction in which a carbon–carbon bond is formed between the carbonyl groups of an aldehyde or a ketone in presence of an electron donor in a free radical process. The reaction product is a vicinal diol. The reaction is named after pinacol, which is the product of this reaction when done with acetone as reagent. The reaction is usually a homocoupling but intramolecular cross-coupling reactions are also possible. Pinacol was discovered by Wilhelm Rudolph Fittig in 1859.

The Johnson–Corey–Chaykovsky reaction is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and cyclopropanes. It was discovered in 1961 by A. William Johnson and developed significantly by E. J. Corey and Michael Chaykovsky. The reaction involves addition of a sulfur ylide to a ketone, aldehyde, imine, or enone to produce the corresponding 3-membered ring. The reaction is diastereoselective favoring trans substitution in the product regardless of the initial stereochemistry. The synthesis of epoxides via this method serves as an important retrosynthetic alternative to the traditional epoxidation reactions of olefins.
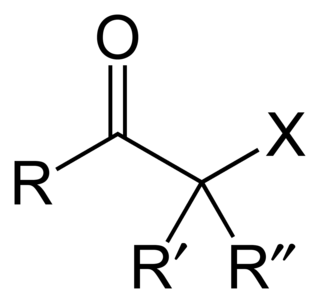
In organic chemistry, an α-halo ketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-Halo ketones are alkylating agents. Prominent α-halo ketones include phenacyl bromide and chloroacetone.
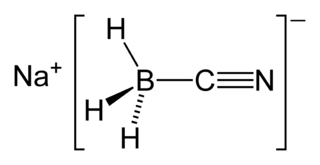
Sodium cyanoborohydride is a chemical compound with the formula Na[BH3(CN)]. It is a colourless salt used in organic synthesis for chemical reduction including that of imines and carbonyls. Sodium cyanoborohydride is a milder reductant than other conventional reducing agents.

Danishefsky's diene is an organosilicon compound and a diene with the formal name trans-1-methoxy-3-trimethylsilyloxy-buta-1,3-diene named after Samuel J. Danishefsky. Because the diene is very electron-rich it is a very reactive reagent in Diels-Alder reactions. This diene reacts rapidly with electrophilic alkenes, such as maleic anhydride. The methoxy group promotes highly regioselective additions. The diene is known to react with amines, aldehydes, alkenes and alkynes. Reactions with imines and nitro-olefins have been reported.
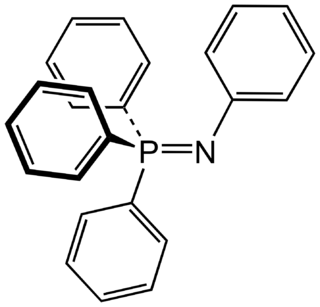
Iminophosphorane is a kind of organophosphorus compound with the formula R3PNR'. Like the corresponding phosphine oxides and Wittig reagents, iminophosphoranes are ylides. Their bonding is described by two resonance structures.

In organic chemistry, the Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde or formate. The reaction was discovered by Teruaki Mukaiyama in 1973. His choice of reactants allows for a crossed aldol reaction between an aldehyde and a ketone, or a different aldehyde without self-condensation of the aldehyde. For this reason the reaction is used extensively in organic synthesis.
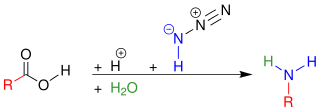
In organic chemistry, the Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl derivative, usually an aldehyde, ketone, or carboxylic acid, under acidic conditions to give an amine or amide, with expulsion of nitrogen. It is named after Karl Friedrich Schmidt (1887–1971), who first reported it in 1924 by successfully converting benzophenone and hydrazoic acid to benzanilide. The intramolecular reaction was not reported until 1991 but has become important in the synthesis of natural products. The reaction is effective with carboxylic acids to give amines (above), and with ketones to give amides (below).
The Bucherer–Bergs reaction is the chemical reaction of carbonyl compounds or cyanohydrins with ammonium carbonate and potassium cyanide to give hydantoins. The reaction is named after Hans Theodor Bucherer.
The Abramov reaction is the related conversions of trialkyl to α-hydroxy phosphonates by the addition to carbonyl compounds. In terms of mechanism, the reaction involves attack of the nucleophilic phosphorus atom on the carbonyl carbon. It was named after the Russian chemist Vasilii Semenovich Abramov (1904–1968) in 1957.
In organic chemistry, the Baylis–Hillman, Morita–Baylis–Hillman, or MBH reaction is a carbon-carbon bond-forming reaction between an activated alkene and a carbon electrophile in the presence of a nucleophilic catalyst, such as a tertiary amine or phosphine. The product is densely functionalized, joining the alkene at the α-position to a reduced form of the electrophile.
The Kirsanov reaction is a method for the synthesis of certain organophosphorus compounds. In this reaction a tertiary phosphine is combined with a halogen and then an amine to give the iminophosphines, which are useful ligands and useful reagents. A typical reaction involves triphenylphosphine with bromine to give bromotriphenylphosphonium bromide:
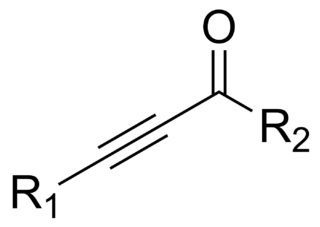
In organic chemistry, an ynone is an organic compound containing a ketone functional group and a C≡C triple bond. Many ynones are α,β-ynones, where the carbonyl and alkyne groups are conjugated. Capillin is a naturally occurring example. Some ynones are not conjugated.
Carbonyl olefin metathesis is a type of metathesis reaction that entails, formally, the redistribution of fragments of an alkene and a carbonyl by the scission and regeneration of carbon-carbon and carbon-oxygen double bonds respectively. It is a powerful method in organic synthesis using simple carbonyls and olefins and converting them into less accessible products with higher structural complexity.












