
Sidney Altman was a Canadian-American molecular biologist, who was the Sterling Professor of Molecular, Cellular, and Developmental Biology and Chemistry at Yale University. In 1989, he shared the Nobel Prize in Chemistry with Thomas R. Cech for their work on the catalytic properties of RNA.

Stanley Ben Prusiner is an American neurologist and biochemist. He is the Director of the Institute for Neurodegenerative Diseases at University of California, San Francisco (UCSF). Prusiner discovered prions, a class of infectious self-reproducing pathogens primarily or solely composed of protein. He received the Albert Lasker Award for Basic Medical Research in 1994 and the Nobel Prize in Physiology or Medicine in 1997 for prion research developed by him and his team of experts beginning in the early 1970s.

James Batcheller Sumner was an American chemist. He discovered that enzymes can be crystallized, for which he shared the Nobel Prize in Chemistry in 1946 with John Howard Northrop and Wendell Meredith Stanley. He was also the first to prove that enzymes are proteins.

Julius Axelrod was an American biochemist. He won a share of the Nobel Prize in Physiology or Medicine in 1970 along with Bernard Katz and Ulf von Euler. The Nobel Committee honored him for his work on the release and reuptake of catecholamine neurotransmitters, a class of chemicals in the brain that include epinephrine, norepinephrine, and, as was later discovered, dopamine. Axelrod also made major contributions to the understanding of the pineal gland and how it is regulated during the sleep-wake cycle.

Daniel Nathans was an American microbiologist. He shared the 1978 Nobel Prize in Physiology or Medicine for the discovery of restriction enzymes and their application in restriction mapping.

William Howard Stein was an American biochemist who collaborated in the determination of the ribonuclease sequence, as well as how its structure relates to catalytic activity, earning a Nobel Prize in Chemistry in 1972 for his work. Stein was also involved in the invention of the automatic amino acid analyzer, an advancement in chromatography that opened the door to modern methods of chromatography, such as liquid chromatography and gas chromatography.

Stanford Moore was an American biochemist. He shared a Nobel Prize in Chemistry in 1972, with Christian B. Anfinsen and William Howard Stein, for work done at Rockefeller University on the structure of the enzyme ribonuclease and for contributing to the understanding of the connection between the chemical structure and catalytic activity of the ribonuclease molecule.

Martin Rodbell was an American biochemist and molecular endocrinologist who is best known for his discovery of G-proteins. He shared the 1994 Nobel Prize in Physiology or Medicine with Alfred G. Gilman for "their discovery of G-proteins and the role of these proteins in signal transduction in cells."

Arne Wilhelm Kaurin Tiselius was a Swedish biochemist who won the Nobel Prize in Chemistry in 1948 "for his research on electrophoresis and adsorption analysis, especially for his discoveries concerning the complex nature of the serum proteins."

Robert Bruce Merrifield was an American biochemist who won the Nobel Prize in Chemistry in 1984 for the invention of solid phase peptide synthesis.

Martin Karplus is an Austrian and American theoretical chemist. He is the Director of the Biophysical Chemistry Laboratory, a joint laboratory between the French National Center for Scientific Research and the University of Strasbourg, France. He is also the Theodore William Richards Professor of Chemistry, emeritus at Harvard University. Karplus received the 2013 Nobel Prize in Chemistry, together with Michael Levitt and Arieh Warshel, for "the development of multiscale models for complex chemical systems".

Bovine pancreatic ribonuclease, also often referred to as bovine pancreatic ribonuclease A or simply RNase A, is a pancreatic ribonuclease enzyme that cleaves single-stranded RNA. Bovine pancreatic ribonuclease is one of the classic model systems of protein science. Two Nobel Prizes in Chemistry have been awarded in recognition of work on bovine pancreatic ribonuclease: in 1972, the Prize was awarded to Christian Anfinsen for his work on protein folding and to Stanford Moore and William Stein for their work on the relationship between the protein's structure and its chemical mechanism; in 1984, the Prize was awarded to Robert Bruce Merrifield for development of chemical synthesis of proteins.

Frederic Middlebrook Richards, commonly referred to as Fred Richards, was an American biochemist and biophysicist known for solving the pioneering crystal structure of the ribonuclease S enzyme in 1967 and for defining the concept of solvent-accessible surface. He contributed many key experimental and theoretical results and developed new methods, garnering over 20,000 journal citations in several quite distinct research areas. In addition to the protein crystallography and biochemistry of ribonuclease S, these included solvent accessibility and internal packing of proteins, the first side-chain rotamer library, high-pressure crystallography, new types of chemical tags such as biotin/avidin, the nuclear magnetic resonance (NMR) chemical shift index, and structural and biophysical characterization of the effects of mutations.
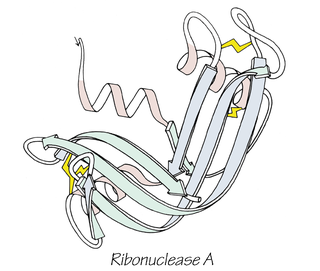
Anfinsen's dogma, also known as the thermodynamic hypothesis, is a postulate in molecular biology. It states that, at least for a small globular protein in its standard physiological environment, the native structure is determined only by the protein's amino acid sequence. The dogma was championed by the Nobel Prize Laureate Christian B. Anfinsen from his research on the folding of ribonuclease A. The postulate amounts to saying that, at the environmental conditions at which folding occurs, the native structure is a unique, stable and kinetically accessible minimum of the free energy. In other words, there are three conditions for formation of a unique protein structure:

Donald Sharp "Don" Fredrickson was an American medical researcher, principally of the lipid and cholesterol metabolism, and director of National Institutes of Health and subsequently the Howard Hughes Medical Institute.

Kathryn C. Zoon is a U.S.-based immunologist, elected to the U.S. Institute of Medicine in 2002 for her research on human interferons. She is the former scientific director of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) in Bethesda, Maryland. From 1992 to 2002, Zoon was director of the FDA Center for Biologics Evaluation and Research (CBER).

Martha Vaughan was an American biochemist at the National Heart Lung and Blood Institute (NHLBI), part of the National Institutes of Health (NIH) in Bethesda, Maryland. She holds the title of emeritus scientist in the Laboratory of Metabolic Regulation and previously served as chief of NHLBI’s Laboratory of Cellular Metabolism. At the NIH, much of her work has focused on cell signaling, cellular regulation, lipid metabolism, and the identification of key proteins associated with cholera toxin and pertussis toxin. Vaughan first came to the NIH in the agency’s fledgling National Heart Institute, now NHLBI, and with the title of senior assistant surgeon worked on protein synthesis in the Building 3 laboratory of biochemist and public scientist Christian B. Anfinsen, Ph.D., who went on to share the 1972 Nobel Prize in Chemistry

Gregg Leonard Semenza is a Pediatrician and Professor of Genetic Medicine at the Johns Hopkins School of Medicine. He serves as the director of the vascular program at the Institute for Cell Engineering. He is a 2016 recipient of the Albert Lasker Award for Basic Medical Research. He is known for his discovery of HIF-1, which allows cancer cells to adapt to oxygen-poor environments. He shared the 2019 Nobel Prize in Physiology or Medicine for "discoveries of how cells sense and adapt to oxygen availability" with William Kaelin Jr. and Peter J. Ratcliffe.
Barry H. Honig is an American biochemist, molecular biophysicist, and computational biophysicist, who develops theoretical methods and computer software for "analyzing the structure and function of biological macromolecules."
William F. Harrington was an American biochemist known for his work on the structure and function of myosins and collagen.





















