
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

Bisulfide is an inorganic anion with the chemical formula HS−. It contributes no color to bisulfide salts, and its salts may have a distinctive putrid smell. It is a strong base. Bisulfide solutions are corrosive and attack the skin.

Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electropositivity of all known elements and fluorine has the highest electronegativity of all known elements.
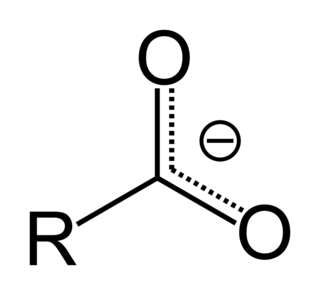
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, RCOO−. It is an ion with negative charge.
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2.

Tetrafluoroborate is the anion BF−
4. This tetrahedral species is isoelectronic with tetrafluoroberyllate (BeF2−
4), tetrafluoromethane (CF4), and tetrafluoroammonium (NF+
4) and is valence isoelectronic with many stable and important species including the perchlorate anion, ClO−
4, which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF3, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid.

Dimethyl acetylenedicarboxylate (DMAD) is an organic compound with the formula CH3O2CC2CO2CH3. It is a di-ester in which the ester groups are conjugated with a C-C triple bond. As such, the molecule is highly electrophilic, and is widely employed as a dienophile in cycloaddition reactions, such as the Diels-Alder reaction. It is also a potent Michael acceptor. This compound exists as a colorless liquid at room temperature. This compound was used in the preparation of nedocromil.
Thiocarbonate describes a family of anions with the general chemical formula CS
3−xO2−
x (x = 0, 1, or 2):

Tetrahydroxy-1,4-benzoquinone, also called tetrahydroxy-p-benzoquinone, tetrahydroxybenzoquinone, or tetrahydroxyquinone, is an organic compound with formula C6O2(OH)4. Its molecular structure consists of a cyclohexadiene ring with four hydroxyl groups and two ketone groups in opposite (para) positions.
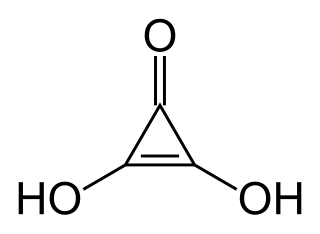
Deltic acid is a chemical substance with the chemical formula C3O(OH)2. It can be viewed as a ketone and double enol of cyclopropene. At room temperature, it is a stable white solid, soluble in diethyl ether, that decomposes between 140 °C and 180 °C, and reacts slowly with water.

Croconic acid is a chemical compound with formula C5H2O5 or (C=O)3(COH)2. It has a cyclopentene backbone with two hydroxyl groups adjacent to the double bond and three ketone groups on the remaining carbon atoms. It is sensitive to light, soluble in water and ethanol and forms yellow crystals that decompose at 212 °C.
In chemistry, an oxocarbon anion is a negative ion consisting solely of carbon and oxygen atoms, and therefore having the general formula C
xOn−
y for some integers x, y, and n.

In chemistry, peroxydicarbonate is a divalent anion with the chemical formula C
2O2−
6. It is one of the oxocarbon anions, which consist solely of carbon and oxygen. Its molecular structure can be viewed as two carbonate anions joined so as to form a peroxide bridge –O–O–.

In chemistry, peroxycarbonate or percarbonate is a divalent anion with formula CO2−
4. It is an oxocarbon anion that consists solely of carbon and oxygen. It would be the anion of a hypothetical peroxycarbonic acid HO–CO–O–OH. or the real hydroperoxyformic acid, HO-O-CO-OH.

Croconate violet or 1,3-bis(dicyanomethylene)croconate is a divalent anion with chemical formula C
11N
4O2−
3 or ((N≡C−)2C=)2(C5O3)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of two oxygen atoms by dicyanomethylene groups =C(−C≡N)2. Its systematic name is 3,5-bis(dicyanomethylene)-1,2,4-trionate. The term croconate violet as a dye name specifically refers to the dipotassium salt K
2C
11N
4O
3.

1,3-Bis(dicyanomethylene)squarate is a divalent anion with chemical formula C
10N
4O2−
2 or ((N≡C−)2C=)2(C4O2)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the squarate oxocarbon anion C
4O2−
4 through the replacement of two opposite oxygen atoms by dicyanomethylene groups =C(−C≡N)2.
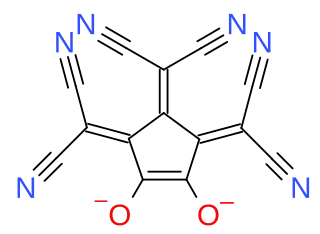
Croconate blue or 1,2,3-tris(dicyanomethylene)croconate is a divalent anion with chemical formula C
14N
6O2−
2 or ((N≡C−)2C=)3(C5O2)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of three oxygen atoms by dicyanomethylene groups =C(−C≡N)2. The term Croconate Blue as a dye name specifically refers to the dipotassium salt K
2C
14N
6O
2.
Azidotetrazolate (CN7−) is an anion which forms a highly explosive series of salts. The ion is made by removing a proton from 5-azido-1H-tetrazole. The molecular structure contains a five-membered ring with four nitrogen atoms, and an azido side chain connected to the carbon atom. Several salts exist, but they are unstable and spontaneously explode. Rubidium azidotetrazolate was so unstable that it explodes while crystallizing. The potassium and caesium salt also spontaneously explode when dry.

Hydromelonic acid, is an elusive chemical compound with formula C
9H
3N
13 or (HNCN)
3(C
6N
7), whose molecule would consist of a heptazine H3(C
6N
7) molecule, with three cyanamido groups H–N=C=N– or N≡C–NH– substituted for the hydrogen atoms.






















