| |
 | |
| Identifiers | |
|---|---|
| |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.037.470 |
| EC Number |
|
| 846955 | |
PubChem CID | |
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| Ba(OH)2 | |
| Molar mass | 171.34 g/mol (anhydrous) 189.355 g/mol (monohydrate) 315.46 g/mol (octahydrate) |
| Appearance | white solid |
| Density | 3.743 g/cm3 (monohydrate) 2.18 g/cm3 (octahydrate, 16 °C) |
| Melting point | 78 °C (172 °F; 351 K) (octahydrate) 300 °C (monohydrate) 407 °C (anhydrous) |
| Boiling point | 780 °C (1,440 °F; 1,050 K) |
| mass of BaO (not Ba(OH)2): 1.67 g/100 mL (0 °C) 3.89 g/100 mL (20 °C) 4.68 g/100 mL (25 °C) 5.59 g/100 mL (30 °C) 8.22 g/100 mL (40 °C) 11.7 g/100 mL (50 °C) 20.94 g/100 mL (60 °C) 101.4 g/100 mL (100 °C)[ citation needed ] | |
| Solubility in other solvents | low |
| Basicity (pKb) | 0.15 (first OH–), 0.64 (second OH–) [1] |
| −53.2·10−6 cm3/mol | |
Refractive index (nD) | 1.50 (octahydrate) |
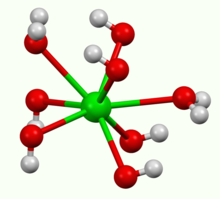
| Structure | |
| octahedral | |
| Thermochemistry [2] | |
Std enthalpy of formation (ΔfH⦵298) | −944.7 kJ·mol−1 |
Enthalpy of fusion (ΔfH⦵fus) | 16 kJ·mol−1 |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H302, H314, H332, H412 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 308 mg/kg (rat, oral) |
| Safety data sheet (SDS) | Fisher Scientific |
| Related compounds | |
Other anions | Barium oxide Barium peroxide |
Other cations | Calcium hydroxide Strontium hydroxide |
| Supplementary data page | |
| Barium hydroxide (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (x = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form.