
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the lanthanides. It was discovered in 1879 by spectral analysis of the minerals euxenite and gadolinite from Scandinavia.
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent solubility product which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios.

In chemistry, iron(III) refers to the element iron in its +3 oxidation state. In ionic compounds (salts), such an atom may occur as a separate cation (positive ion) denoted by Fe3+.

Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash.

Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca(OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide.

Iron(II) hydroxide or ferrous hydroxide is an inorganic compound with the formula Fe(OH)2. It is produced when iron(II) salts, from a compound such as iron(II) sulfate, are treated with hydroxide ions. Iron(II) hydroxide is a white solid, but even traces of oxygen impart a greenish tinge. The air-oxidised solid is sometimes known as "green rust".

Scandium(III) chloride is the inorganic compound with the formula ScCl3. It is a white, high-melting ionic compound, which is deliquescent and highly water-soluble. This salt is mainly of interest in the research laboratory. Both the anhydrous form and hexahydrate (ScCl3•6H2O) are commercially available.

Silver oxide is the chemical compound with the formula Ag2O. It is a fine black or dark brown powder that is used to prepare other silver compounds.
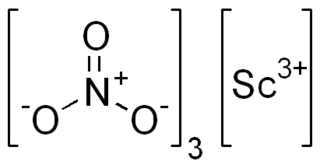
Scandium(III) nitrate, Sc(NO3)3, is an ionic compound. It is an oxidizer, as all nitrates are. The salt is applied in optical coatings, catalysts, electronic ceramics and the laser industry.

Scandium(III) fluoride, ScF3, is an ionic compound. This salt is slightly soluble in water but dissolves in the presence of excess fluoride to form the ScF63− anion.

Zinc fluoride is an inorganic chemical compound with the chemical formula ZnF2. It is encountered as the anhydrous form and also as the tetrahydrate, ZnF2·4H2O (rhombohedral crystal structure). It has a high melting point and has the rutile structure containing 6 coordinate zinc, which suggests appreciable ionic character in its chemical bonding. Unlike the other zinc halides, ZnCl2, ZnBr2 and ZnI2, it is not very soluble in water.
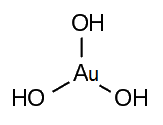
Gold(III) hydroxide, gold trihydroxide, or gold hydroxide is an inorganic compound, a hydroxide of gold, with formula Au(OH)3. It is also called auric acid with formula H3AuO3. It is easily dehydrated above 140 °C to gold(III) oxide. Salts of auric acid are termed aurates.
Scandium compounds are compounds containing the element scandium. The chemistry of scandium is almost completely dominated by the trivalent ion, Sc3+, due to its electron configuration, [Ar] 3d14s2. The radii of M3+ ions in the table below indicate that the chemical properties of scandium ions have more in common with yttrium ions than with aluminium ions. In part because of this similarity, scandium is often classified as a lanthanide-like element.
Germanium(II) hydroxide, normally written as Ge(OH)2, is a poorly characterised compound, sometimes called hydrous germanium(II) oxide or germanous hydroxide. It was first reported by Winkler in 1886.
An yttrium compound is a chemical compound containing yttrium. Among these compounds, yttrium generally has a +3 valence. The solubility properties of yttrium compounds are similar to those of the lanthanides. For example oxalates and carbonates are hardly soluble in water, but soluble in excess oxalate or carbonate solutions as complexes are formed. Sulfates and double sulfates are generally soluble. They resemble the "yttrium group" of heavy lanthanide elements.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Lutetium compounds are compounds formed by the lanthanide metal lutetium (Lu). In these compounds, lutetium generally exhibits the +3 oxidation state, such as LuCl3, Lu2O3 and Lu2(SO4)3. Aqueous solutions of most lutetium salts are colorless and form white crystalline solids upon drying, with the common exception of the iodide. The soluble salts, such as nitrate, sulfate and acetate form hydrates upon crystallization. The oxide, hydroxide, fluoride, carbonate, phosphate and oxalate are insoluble in water.

Technetium(IV) oxide, also known as technetium dioxide, is a chemical compound with the formula TcO2 which forms the dihydrate, TcO2·2H2O, which is also known as technetium(IV) hydroxide. It is a radioactive black solid which slowly oxidizes in air.
Scandium acetate is an inorganic compound, with the chemical formula of Sc(CH3COO)3. It exists in the anhydrous and the hydrate forms. It can be obtained by reacting scandium hydroxide with aqueous acetic acid. It is a water-soluble crystal that decomposes into scandium oxide at high temperature. It can be used to prepare other scandium-containing materials.













