Barium is a chemical element; it has symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.

Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48-52%) but there are also stronger solutions and pure HF has a boiling point near room temperature. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers.

Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.

Barium nitrate is the inorganic compound with the chemical formula Ba(NO3)2. It, like most barium salts, is colorless, toxic, and water-soluble. It burns with a green flame and is an oxidizer; the compound is commonly used in pyrotechnics.

Sodium fluoride (NaF) is an inorganic compound with the formula NaF. It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes and topical pharmaceuticals for the same purpose. In 2021, it was the 291st most commonly prescribed medication in the United States, with more than 600,000 prescriptions. It is also used in metallurgy and in medical imaging.

Hydrogen fluoride (fluorane) is an inorganic compound with chemical formula HF. It is a very poisonous, colorless gas or liquid that dissolves in water to yield an aqueous solution termed hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils at near room temperature, much higher than other hydrogen halides.

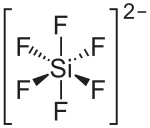
Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Silicon tetrafluoride or tetrafluorosilane is a chemical compound with the formula SiF4. This colorless gas is notable for having a narrow liquid range: its boiling point is only 4 °C above its melting point. It was first prepared in 1771 by Carl Wilhelm Scheele by dissolving silica in hydrofluoric acid., later synthesized by John Davy in 1812. It is a tetrahedral molecule and is corrosive.

Barium peroxide is an inorganic compound with the formula BaO2. This white solid is one of the most common inorganic peroxides, and it was the first peroxide compound discovered. Being an oxidizer and giving a vivid green colour upon ignition, it finds some use in fireworks; historically, it was also used as a precursor for hydrogen peroxide.

Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic. It is isostructural with BaSO4, and contains the tetrahedral [FeO4]2− anion.

Barium borate is an inorganic compound, a borate of barium with a chemical formula BaB2O4 or Ba(BO2)2. It is available as a hydrate or dehydrated form, as white powder or colorless crystals. The crystals exist in the high-temperature α phase and low-temperature β phase, abbreviated as BBO; both phases are birefringent, and BBO is a common nonlinear optical material.

Bararite is a natural form of ammonium fluorosilicate (also known as hexafluorosilicate or fluosilicate). It has chemical formula (NH4)2SiF6 and trigonal crystal structure. This mineral was once classified as part of cryptohalite. Bararite is named after the place where it was first described, Barari, India. It is found at the fumaroles of volcanoes (Vesuvius, Italy), over burning coal seams (Barari, India), and in burning piles of anthracite (Pennsylvania, U.S.). It is a sublimation product that forms with cryptohalite, sal ammoniac, and native sulfur.
Ammonium fluorosilicate (also known as ammonium hexafluorosilicate, ammonium fluosilicate or ammonium silicofluoride) has the formula (NH4)2SiF6. It is a toxic chemical, like all salts of fluorosilicic acid. It is made of white crystals, which have at least three polymorphs and appears in nature as rare minerals cryptohalite or bararite.
Nickel compounds are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.

Metal peroxides are metal-containing compounds with ionically- or covalently-bonded peroxide (O2−
2) groups. This large family of compounds can be divided into ionic and covalent peroxide. The first class mostly contains the peroxides of the alkali and alkaline earth metals whereas the covalent peroxides are represented by such compounds as hydrogen peroxide and peroxymonosulfuric acid (H2SO5). In contrast to the purely ionic character of alkali metal peroxides, peroxides of transition metals have a more covalent character.
Sodium hexafluoroarsenate is an inorganic chemical compound with the chemical formula NaAsF6.
Potassium hexafluoroantimonate is an inorganic chemical compound with the chemical formula KSbF6.

Potassium hexafluoroaluminate is an inorganic chemical compound with the chemical formula K3AlF6. It naturally occures as the mineral cryolite.
Barium hexafluorogermanate is an inorganic chemical compound with the chemical formula BaGeF6.
Lithium hexafluorosilicate is an inorganic chemical compound with the chemical formula Li2SiF6.