Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.

Hydrogen fluoride (fluorane) is an inorganic compound with chemical formula HF. It is a very poisonous, colorless gas or liquid that dissolves in water to yield an aqueous solution termed hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils at near room temperature, much higher than other hydrogen halides.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Ammonium bifluoride is the inorganic compound with the formula [NH4][HF2] or [NH4]F·HF. It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-etchant and an intermediate in a once-contemplated route to hydrofluoric acid.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.

Silicon tetrafluoride or tetrafluorosilane is a chemical compound with the formula SiF4. This colorless gas is notable for having a narrow liquid range: its boiling point is only 4 °C above its melting point. It was first prepared in 1771 by Carl Wilhelm Scheele by dissolving silica in hydrofluoric acid., later synthesized by John Davy in 1812. It is a tetrahedral molecule and is corrosive.

Aluminium fluoride is an inorganic compound with the formula AlF3. It forms hydrates AlF3·xH2O. Anhydrous AlF3 and its hydrates are all colorless solids. Anhydrous AlF3 is used in the production of aluminium. Several occur as minerals.

Zirconium(IV) fluoride describes members of a family inorganic compounds with the formula (ZrF4(H2O)x. All are colorless, diamagnetic solids. Anhydrous Zirconium(IV) fluoride' is a component of ZBLAN fluoride glass.

Technetium hexafluoride or technetium(VI) fluoride (TcF6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium (spontaneous fission in natural uranium, possible contamination from induced fission inside the reactor in reprocessed uranium). The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing.

Bismuth(III) fluoride or bismuth trifluoride is a chemical compound of bismuth and fluorine. The chemical formula is BiF3. It is a grey-white powder melting at 649 °C.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.
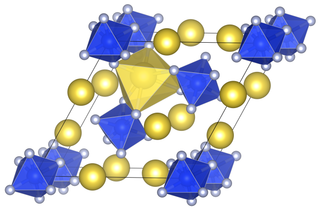
Sodium fluorosilicate is a compound with the chemical formula Na2[SiF6]. Unlike other sodium salts, it has a low solubility in water.
Dinitrogen difluoride is a chemical compound with the formula N2F2. It is a gas at room temperature, and was first identified in 1952 as the thermal decomposition product of the fluorine azide. It has the structure F−N=N−F and exists in both cis and trans isomers, as typical for diimides.
Ammonium fluorosilicate (also known as ammonium hexafluorosilicate, ammonium fluosilicate or ammonium silicofluoride) has the formula (NH4)2SiF6. It is a toxic chemical, like all salts of fluorosilicic acid. It is made of white crystals, which have at least three polymorphs and appears in nature as rare minerals cryptohalite or bararite.

Rhenium hexafluoride, also rhenium(VI) fluoride, (ReF6) is a compound of rhenium and fluorine and one of the seventeen known binary hexafluorides.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Potassium hexafluorotitanate is an inorganic compound of potassium, fluorine, and titanium with the chemical formula K2TiF6.
Lithium hexafluorotitanate is an inorganic compound of lithium, fluorine, and titanium with the chemical formula Li2TiF6.
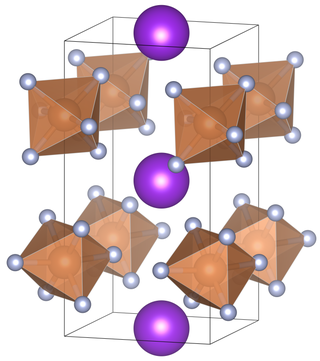
Potassium hexafluoroantimonate is an inorganic chemical compound with the chemical formula KSbF6.












