
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula CH3. In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond, it can be found on its own in any of three forms: methanide anion, methylium cation or methyl radical. The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed.
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure ionic bonding, many covalent bonds exhibit a strong ionicity, making oxidation state a useful predictor of charge.

In chemistry, there are three definitions in common use of the word "base": Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.

In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen atom with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and more frequently in the past) been applied to all compounds containing covalently bound H atoms. In this broad and potentially archaic sense, water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. In covalent compounds, it implies hydrogen is attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.

A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other commercial rechargeable batteries, Li-ion batteries are characterized by higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991: over the following 30 years, their volumetric energy density increased threefold while their cost dropped tenfold.
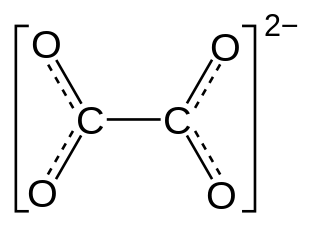
Oxalate is an anion with the chemical formula C2O2−4. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate, and several esters such as dimethyl oxalate. It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate.

Bisulfide is an inorganic anion with the chemical formula HS−. It contributes no color to bisulfide salts, and its salts may have a distinctive putrid smell. It is a strong base. Bisulfide solutions are corrosive and attack the skin.

In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .
The proton affinity of an anion or of a neutral atom or molecule is the negative of the enthalpy change in the reaction between the chemical species concerned and a proton in the gas phase:

Tetrahydroxy-1,4-benzoquinone, also called tetrahydroxy-p-benzoquinone, tetrahydroxybenzoquinone, or tetrahydroxyquinone, is an organic compound with formula C6O2(OH)4. Its molecular structure consists of a cyclohexadiene ring with four hydroxyl groups and two ketone groups in opposite (para) positions.
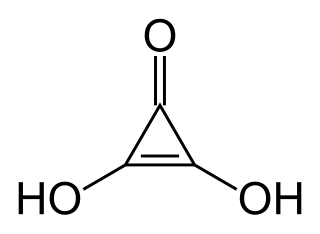
Deltic acid is a chemical substance with the chemical formula C3O(OH)2. It can be viewed as a ketone and double enol of cyclopropene. At room temperature, it is a stable white solid, soluble in diethyl ether, that decomposes between 140 °C and 180 °C, and reacts slowly with water.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.

The Birch reduction is an organic reaction that is used to convert arenes to 1,4-cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent with an alkali metal and a proton source. Unlike catalytic hydrogenation, Birch reduction does not reduce the aromatic ring all the way to a cyclohexane.

The carbonite ion is an anion with the chemical formula CO2−2. This divalent anion forms by deprotonation of carbonous acid. Alkali metal salts of carbonous acid, Li2CO2, K2CO2, Rb2CO2 and Cs2CO2, have been observed at 15 K. Interestingly, the disodium salt has not been directly observed under experimental conditions, suggesting that this is less stable than other alkali carbonites. Due to the lone pair on the carbon atom, salts of the carbonite ion would be protonated to form formate and formic acid, rather than the carbene.

In organic chemistry, a diethynylbenzene dianion is an anion consisting of two ethynyl anions as substituents on a benzene ring. With the chemical formula C
6H
4C2−
4, three positional isomers are possible, differing in the relative positions of the two substituents around the ring:
Arumugam Manthiram is an Indian-American materials scientist and engineer, best known for his identification of the polyanion class of lithium-ion battery cathodes, understanding of how chemical instability limits the capacity of layered oxide cathodes, and technological advances in lithium sulfur batteries. He is a Cockrell Family Regents Chair in engineering, Director of the Texas Materials Institute, the Director of the Materials Science and Engineering Program at the University of Texas at Austin, and a former lecturer of Madurai Kamaraj University. Manthiram delivered the 2019 Nobel Lecture in Chemistry on behalf of Chemistry Laureate John B. Goodenough.
The borate oxalates are chemical compounds containing borate and oxalate anions. Where the oxalate group is bound to the borate via oxygen, a more condensed anion is formed that balances less cations. These can be termed boro-oxalates, bis(oxalato)borates, or oxalatoborates or oxalate borates. The oxalatoborates are heterocyclic compounds with a ring containing -O-B-O-. Bis(oxalato)borates are spiro compounds with rings joined at the boron atom.

Lithium naphthalene is an organic salt with the chemical formula Li+[C10H8]−. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. Lithium naphthalene crystallizes with ligands bound to Li+. The anion is a well-known example of an organic radical.

Superelectrophilic anions are a class of molecular ions that exhibit highly electrophilic reaction behavior despite their overall negative charge. Thus, they are even able to bind the unreactive noble gases or molecular nitrogen at room temperature. The only representatives known so far are the fragment ions of the type [B12X11]– derived from the closo-dodecaborate dianions [B12X12]2–. X represents a substituent connected to a boron atom (cf. Fig. 1). For this reason, the following article deals exclusively with superelectrophilic anions of this type.













