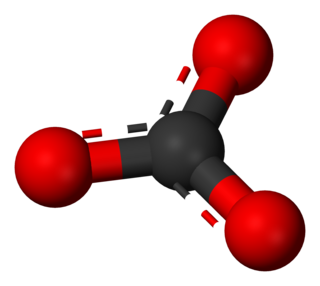
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula of CO2−
3. The name may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2.

Lithium carbonate is an inorganic compound, the lithium salt of carbonate with the formula Li
2CO
3. This white salt is widely used in the processing of metal oxides, and as a drug for the treatment of mood disorders.

Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation (Na+) and a bicarbonate anion (HCO3−). Sodium bicarbonate is a white solid that is crystalline, but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite. It is a component of the mineral natron and is found dissolved in many mineral springs.

A Gilman reagent is a lithium and copper (diorganocopper) reagent compound, R2CuLi, where R is an alkyl or aryl. These reagents are useful because, unlike related Grignard reagents and organolithium reagents, they react with organic halides to replace the halide group with an R group (the Corey–House reaction). Such displacement reactions allow for the synthesis of complex products from simple building blocks.

Organolithium reagents are organometallic compounds that contain carbon – lithium bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C-Li bond is highly ionic. Owing to the polar nature of the C-Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.
Calcium bicarbonate, also called calcium hydrogencarbonate, has the chemical formula Ca(HCO3)2. The term does not refer to a known solid compound; it exists only in aqueous solution containing calcium (Ca2+), bicarbonate (HCO−
3), and carbonate (CO2−
3) ions, together with dissolved carbon dioxide (CO2). The relative concentrations of these carbon-containing species depend on the pH; bicarbonate predominates within the range 6.36–10.25 in fresh water.
Pentazole is an aromatic chemical molecule consisting of a five-membered ring with all nitrogen atoms, one of which is bonded to a hydrogen atom. It has a molecular formula HN5. Although strictly speaking a homocyclic, inorganic compound, pentazole has historically been classed as the last in a series of heterocyclic azole compounds containing one to five nitrogen atoms. This set contains pyrrole, imidazole, pyrazole, triazoles, tetrazole, and pentazole.
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.

Lithium bromide (LiBr) is a chemical compound of lithium and bromine. Its extreme hygroscopic character makes LiBr useful as a desiccant in certain air conditioning systems.
PubChem is a database of chemical molecules and their activities against biological assays. The system is maintained by the National Center for Biotechnology Information (NCBI), a component of the National Library of Medicine, which is part of the United States National Institutes of Health (NIH). PubChem can be accessed for free through a web user interface. Millions of compound structures and descriptive datasets can be freely downloaded via FTP. PubChem contains multiple substance descriptions and small molecules with fewer than 100 atoms and 1000 bonds. More than 80 database vendors contribute to the growing PubChem database.
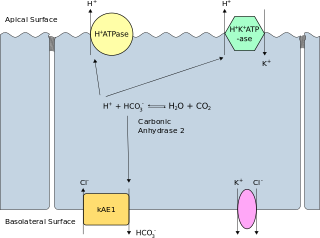
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the SLC4A1 gene in humans.
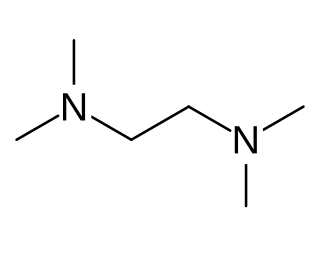
Tetramethylethylenediamine (TMEDA or TEMED) is a chemical compound with the formula (CH3)2NCH2CH2N(CH3)2. This species is derived from ethylenediamine by replacement of the four amine hydrogens with four methyl groups. It is a colorless liquid, although old samples often appear yellow. Its odor is similar to that of rotting fish.

Lithium peroxide is the inorganic compound with the formula Li2O2. It is a white, nonhygroscopic solid. Because of its high oxygen:mass and oxygen:volume ratios, the solid has been used to remove CO2 from the atmosphere in spacecraft.
Acetone cyanohydrin (ACH) is an organic compound used in the production of methyl methacrylate, the monomer of the transparent plastic polymethyl methacrylate (PMMA), also known as acrylic. It liberates hydrogen cyanide easily, so it is used as a source of such. For this reason, this cyanohydrin is also highly toxic.

Sodium bicarbonate cotransporter 3 is a protein which in humans is encoded by the SLC4A7 gene.

Electrogenic sodium bicarbonate cotransporter 1 (NBCe1) is a membrane transport protein that in humans is encoded by the 'SLC4A4' gene.
Sodium citrate may refer to any of the sodium salts of citric acid :
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.

Magnesium bicarbonate or magnesium hydrogencarbonate, Mg(HCO3)2, is the bicarbonate salt of magnesium. It can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide (milk of magnesia).

Vinyllithium is an organolithium compound with the formula LiC2H3. A colorless or white solid, it is encountered mainly as a solution in tetrahydrofuran (THF). It is a reagent in synthesis of organic compounds.













