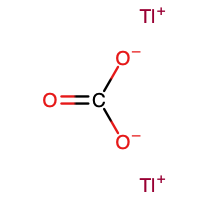Lithium carbonate is an inorganic compound, the lithium salt of carbonic acid with the formula Li
2CO
3. This white salt is widely used in processing metal oxides. It is on the World Health Organization's List of Essential Medicines for its efficacy in the treatment of mood disorders such as bipolar disorder.
Thallium is a chemical element; it has symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861, in residues of sulfuric acid production. Both used the newly developed method of flame spectroscopy, in which thallium produces a notable green spectral line. Thallium, from Greek θαλλός, thallós, meaning "green shoot" or "twig", was named by Crookes. It was isolated by both Lamy and Crookes in 1862; Lamy by electrolysis, and Crookes by precipitation and melting of the resultant powder. Crookes exhibited it as a powder precipitated by zinc at the international exhibition, which opened on 1 May that year.

Barium carbonate is the inorganic compound with the formula BaCO3. Like most alkaline earth metal carbonates, it is a white salt that is poorly soluble in water. It occurs as the mineral known as witherite. In a commercial sense, it is one of the most important barium compounds.

Barium nitrate is the inorganic compound with the chemical formula Ba(NO3)2. It, like most barium salts, is colorless, toxic, and water-soluble. It burns with a green flame and is an oxidizer; the compound is commonly used in pyrotechnics.

Lead(II) chloride (PbCl2) is an inorganic compound which is a white solid under ambient conditions. It is poorly soluble in water. Lead(II) chloride is one of the most important lead-based reagents. It also occurs naturally in the form of the mineral cotunnite.

Thallium(I) sulfate (Tl2SO4) or thallous sulfate is the sulfate salt of thallium in the common +1 oxidation state, as indicated by the Roman numeral I. It is often referred to as simply thallium sulfate.

Manganese carbonate is a compound with the chemical formula MnCO3. Manganese carbonate occurs naturally as the mineral rhodochrosite but it is typically produced industrially. It is a pale pink, water-insoluble solid. Approximately 20,000 metric tonnes were produced in 2005.

Tetrafluoroborate is the anion BF−
4. This tetrahedral species is isoelectronic with tetrafluoroberyllate (BeF2−
4), tetrafluoromethane (CF4), and tetrafluoroammonium (NF+
4) and is valence isoelectronic with many stable and important species including the perchlorate anion, ClO−
4, which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF3, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 CFR 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.
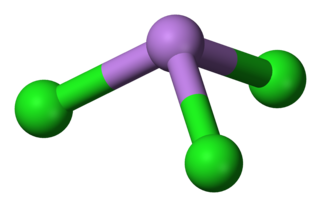
Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.

Calcium arsenate is the inorganic compound with the formula Ca3(AsO4)2. A colourless salt, it was originally used as a pesticide and as a germicide. It is highly soluble in water, in contrast to lead arsenate, which makes it more toxic. Two minerals are hydrates of calcium arsenate: rauenthalite Ca3(AsO4)2·10H2O and phaunouxite Ca3(AsO4)2·11H2O. A related mineral is ferrarisite (Ca5H2(AsO4)4·9H2O.

Thallium(I) bromide is a chemical compound of thallium and bromine with a chemical formula TlBr. This salt is used in room-temperature detectors of X-rays, gamma-rays and blue light, as well as in near-infrared optics.

Thallium(I) chloride, also known as thallous chloride, is a chemical compound with the formula TlCl. This colourless salt is an intermediate in the isolation of thallium from its ores. Typically, an acidic solution of thallium(I) sulfate is treated with hydrochloric acid to precipitate insoluble thallium(I) chloride. This solid crystallizes in the caesium chloride motif.

Sodium ferrocyanide is the sodium salt of the coordination compound of formula [Fe(CN)6]4−. In its hydrous form, Na4Fe(CN)6 · 10 H2O (sodium ferrocyanide decahydrate), it is sometimes known as yellow prussiate of soda. It is a yellow crystalline solid that is soluble in water and insoluble in alcohol. The yellow color is the color of ferrocyanide anion. Despite the presence of the cyanide ligands, sodium ferrocyanide has low toxicity (acceptable daily intake 0–0.025 mg/kg body weight). The ferrocyanides are less toxic than many salts of cyanide, because they tend not to release free cyanide. However, like all ferrocyanide salt solutions, addition of an acid or exposure to UV light can result in the production of hydrogen cyanide gas, which is extremely toxic.
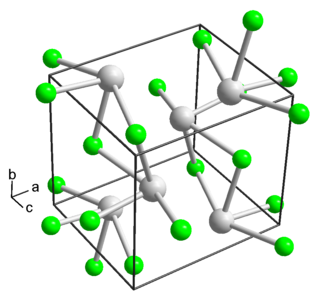
Thallium(I) fluoride is the inorganic compound with the formula TlF. It is a white solid, forming orthorhombic crystals. The solid is slightly deliquescent. It has a distorted sodium chloride (rock salt) crystal structure, due to the 6s2 inert pair on Tl+.

Coumaphos is a nonvolatile, fat-soluble phosphorothioate with ectoparasiticide properties: it kills insects and mites. It is well known by a variety of brand names as a dip or wash, used on farm and domestic animals to control ticks, mites, flies and fleas.
Aluminium carbonate (Al2(CO3)3), is a carbonate of aluminium. It is not well characterized; one authority says that simple carbonates of aluminium are not known. However related compounds are known, such as the basic sodium aluminium carbonate mineral dawsonite (NaAlCO3(OH)2) and hydrated basic aluminium carbonate minerals scarbroite (Al5(CO3)(OH)13•5(H2O)) and hydroscarbroite (Al14(CO3)3(OH)36•nH2O).

Thallous acetate or thallium(I) acetate is a salt of thallium and acetate with the chemical formula TlCH3COO. It is used in microbiology as a selective growth medium. It is poisonous.
Thallous malonate is a chemical compound composed mainly of Thallium. It is an extremely hazardous substance and is on the List of extremely hazardous substances.

Thallium(I) nitrate, also known as thallous nitrate, is a thallium compound with the formula TlNO3. It is a colorless and highly toxic salt.