Praseodymium(IV) oxide is an inorganic compound with chemical formula PrO2.
Dysprosium(III) fluoride is an inorganic compound of dysprosium with a chemical formula DyF3.
Terbium(III) fluoride is an inorganic compound with chemical formula TbF3. It is hard to dissolve in water. It can be produced by reacting terbium(III) carbonate and 40% hydrofluoric acid at 40°C.
Thorium(IV) hydroxide is an inorganic compound with a chemical formula Th(OH)4.

Cerium(IV) hydroxide, also known as ceric hydroxide, is an inorganic compound with the chemical formula Ce(OH)4. It is a yellowish powder that is insoluble in water but soluble in concentrated acids.
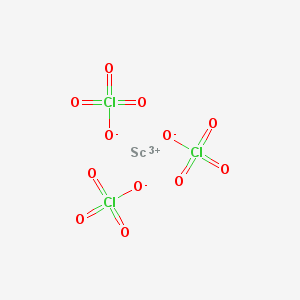
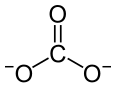
Scandium perchlorate is an inorganic compound with the chemical formula Sc(ClO4)3.
Praseodymium(III) hydroxide is an inorganic compound with a chemical formula Pr(OH)3.

Neodymium(III) hydroxide is an insoluble inorganic compound with the chemical formula Nd(OH)3.

Samarium(III) hydroxide is an inorganic compound with chemical formula Sm(OH)3.

Erbium(III) hydroxide is an inorganic compound with chemical formula Er(OH)3.

Thulium(III) hydroxide is an inorganic compound with the chemical formula Tm(OH)3.

Terbium(III) hydroxide is an inorganic compound with chemical formula Tb(OH)3.

Neodymium compounds are compounds formed by the lanthanide metal neodymium (Nd). In these compounds, neodymium generally exhibits the +3 oxidation state, such as NdCl3, Nd2(SO4)3 and Nd(CH3COO)3. Compounds with neodymium in the +2 oxidation state are also known, such as NdCl2 and NdI2. Some neodymium compounds have colors that vary based upon the type of lighting.
Neodymium(III) carbonate is an inorganic compound, a salt, where neodymium is in the +3 oxidation state and the carbonate ion is in the -2 oxidation state. It has a chemical formula of Nd2(CO3)3. The anhydrous form is purple-red, while the octahydrate is a pink solid. Both of these salts are insoluble in water.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.

Cerium acetate is an inorganic compound with the chemical formula of Ce(CH3COO)3. It is a white powder that is soluble in water. Its 1.5 hydrate loses water at 133°C to obtain an amorphous anhydrous form, and the amorphous phase changes to crystal at 212°C, and phase changes again at 286°C.

Ytterbium(III) iodide is one of ytterbium's iodides, with the chemical formula of YbI3.
A niobate is an oxo-acid salt formed by niobium(V), and the common forms are metaniobate (NbO3−) and orthoniobate (NbO43−). The most common niobates are lithium niobate (LiNbO3) and potassium niobate (KNbO3).
Cerium(III) selenate is an inorganic compound with the chemical formula Ce2(SeO4)3. It can be obtained by reacting selenic acid and cerium(III) carbonate, and the solvent is evaporated to precipitate crystals. The double salt CsCe(SeO4)2·4H2O can be obtained from mixing cerium(III) selenate and cesium selenate in an aqueous solution, and then evaporating and crystallizing the solution.