
Copper(II) nitrate describes any member of the family of inorganic compounds with the formula Cu(NO3)2(H2O)x. The hydrates are blue solids. Anhydrous copper nitrate forms blue-green crystals and sublimes in a vacuum at 150-200 °C. Common hydrates are the hemipentahydrate and trihydrate.
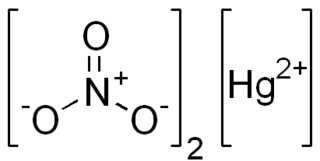
Mercury(II) nitrate is an inorganic compound with the chemical formula Hg(NO3)2. It is the mercury(II) salt of nitric acid HNO3. It contains mercury(II) cations Hg2+ and nitrate anions NO−3, and water of crystallization H2O in the case of a hydrous salt. Mercury(II) nitrate forms hydrates Hg(NO3)2·xH2O. Anhydrous and hydrous salts are colorless or white soluble crystalline solids that are occasionally used as a reagents. Mercury(II) nitrate is made by treating mercury with hot concentrated nitric acid. Neither anhydrous nor monohydrate has been confirmed by X-ray crystallography. The anhydrous material is more widely used.
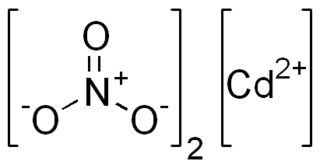
Cadmium nitrate describes any of the related members of a family of inorganic compounds with the general formula Cd(NO3)2·xH2O. The most commonly encountered form being the tetrahydrate.The anhydrous form is volatile, but the others are colourless crystalline solids that are deliquescent, tending to absorb enough moisture from the air to form an aqueous solution. Like other cadmium compounds, cadmium nitrate is known to be carcinogenic. According to X-ray crystallography, the tetrahydrate features octahedral Cd2+ centers bound to six oxygen ligands.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.
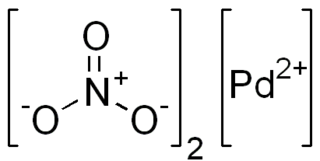
Palladium(II) nitrate is the inorganic compound with the formula Pd(NO3)2.(H2O)x where x = 0 or 2. The anhydrous and dihydrate are deliquescent solids. According to X-ray crystallography, both compounds feature square planar Pd(II) with unidentate nitrate ligands. The anhydrous compound, which is a coordination polymer, is yellow.

Cobalt nitrate is the inorganic compound with the formula Co(NO3)2.xH2O. It is cobalt(II)'s salt. The most common form is the hexahydrate Co(NO3)2·6H2O, which is a red-brown deliquescent salt that is soluble in water and other polar solvents.

Bismuth oxynitrate is the name applied to a number of compounds that contain Bi3+, nitrate ions and oxide ions and which can be considered as compounds formed from Bi2O3, N2O5 and H2O. Other names for bismuth oxynitrate include bismuth subnitrate and bismuthyl nitrate. In older texts bismuth oxynitrate is often simply described as BiONO3 or basic bismuth nitrate. Bismuth oxynitrate was once called magisterium bismuti or bismutum subnitricum, and was used as a white pigment, in beauty care, and as a gentle disinfectant for internal and external use. It is also used to form Dragendorff's reagent, which is used as a TLC stain.

Cerium nitrate refers to a family of nitrates of cerium in the +3 or +4 oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.

Thorium(IV) nitrate is a chemical compound, a salt of thorium and nitric acid with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.

Europium(III) nitrate is an inorganic compound with the formula Eu(NO3)3·x(H2O). The hexahydrate is a common salt. It forms colorless hygroscopic crystals.
Praseodymium(III,IV) oxide is the inorganic compound with the formula Pr6O11 that is insoluble in water. It has a cubic fluorite structure. It is the most stable form of praseodymium oxide at ambient temperature and pressure.
Praseodymium(III) fluoride is an inorganic compound with the formula PrF3, being the most stable fluoride of praseodymium.
Praseodymium(III) hydroxide is an inorganic compound with a chemical formula Pr(OH)3.
Borate nitrates are mixed anion compounds containing separate borate and nitrate anions.

Iron(II) nitrate is the nitrate salt of iron(II). It is commonly encountered as the green hexahydrate, Fe(NO3)2·6H2O, which is a metal aquo complex, however it is not commercially available unlike iron(III) nitrate due to its instability to air. The salt is soluble in water and serves as a ready source of ferrous ions.

Lanthanum(III) nitrate is any inorganic compound with the chemical formula La(NO3)3·xH2O. It is used in the extraction and purification of lanthanum from its ores.

A transition metal nitrate complex is a coordination compound containing one or more nitrate ligands. Such complexes are common starting reagents for the preparation of other compounds.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.
Cerium compounds are compounds containing the element cerium (Ce), a lanthanide. Cerium exists in two main oxidation states, Ce(III) and Ce(IV). This pair of adjacent oxidation states dominates several aspects of the chemistry of this element. Cerium(IV) aqueous solutions may be prepared by reacting cerium(III) solutions with the strong oxidizing agents peroxodisulfate or bismuthate. The value of E⦵(Ce4+/Ce3+) varies widely depending on conditions due to the relative ease of complexation and hydrolysis with various anions, although +1.72 V is representative. Cerium is the only lanthanide which has important aqueous and coordination chemistry in the +4 oxidation state.



















