Praseodymium oxide may refer to:
- Praseodymium(III) oxide (dipraseodymium trioxide), Pr2O3
- Praseodymium(IV) oxide (praseodymium dioxide), PrO2
- Praseodymium(III,IV) oxide, Pr6O11
Praseodymium oxide may refer to:
The lanthanide or lanthanoid series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements.

Neodymium is a chemical element with the symbol Nd and atomic number 60. Neodymium belongs to the lanthanide series and is a rare-earth element. It is a hard, slightly malleable silvery metal that quickly tarnishes in air and moisture. When oxidized, neodymium reacts quickly to produce pink, purple/blue and yellow compounds in the +2, +3 and +4 oxidation states. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach. It is present in significant quantities in the ore minerals monazite and bastnäsite. Neodymium is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Although neodymium is classed as a rare-earth element, it is fairly common, no rarer than cobalt, nickel, or copper, and is widely distributed in the Earth's crust. Most of the world's commercial neodymium is mined in China.
The oxidation state, sometimes referred to as oxidation number, describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component. This is never exactly true for real bonds.

Praseodymium is a chemical element with the symbol Pr and atomic number 59. It is the third member of the lanthanide series and is traditionally considered to be one of the rare-earth metals. Praseodymium is a soft, silvery, malleable and ductile metal, valued for its magnetic, electrical, chemical, and optical properties. It is too reactive to be found in native form, and pure praseodymium metal slowly develops a green oxide coating when exposed to air.
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Copper oxide is a compound from the two elements copper and oxygen.
Manganese oxide is any of a variety of manganese oxides and hydroxides. These include

Praseodymium(III) chloride is the inorganic compound with the formula PrCl3. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green heptahydrate.
Cerium oxide may refer to either of the following:

Terbium(III,IV) oxide, occasionally called tetraterbium heptaoxide, has the formula Tb4O7, though some texts refer to it as TbO1.75. There is some debate as to whether it is a discrete compound, or simply one phase in an interstitial oxide system. Tb4O7 is one of the main commercial terbium compounds, and the only such product containing at least some Tb(IV) (terbium in the +4 oxidation state), along with the more stable Tb(III). It is produced by heating the metal oxalate, and it is used in the preparation of other terbium compounds. Terbium forms three other major oxides: Tb2O3, TbO2, and Tb6O11.

Praseodymium(III) oxide, praseodymium oxide or praseodymia is the chemical compound composed of praseodymium and oxygen with the formula Pr2O3. It forms light green hexagonal crystals. Praseodymium(III) oxide crystallizes in the manganese(III) oxide or bixbyite structure.

Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air, and it is soft enough to be cut with a steel kitchen knife. Cerium is the second element in the lanthanide series, and while it often shows the +3 oxidation state characteristic of the series, it also has a stable +4 state that does not oxidize in water. It is also considered one of the rare-earth elements. Cerium has no biological role in humans and is not very toxic.
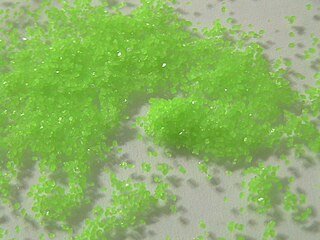
Praseodymium(III) sulfate is a praseodymium compound with formula Pr2(SO4)3. It is an odourless whitish-green crystalline compound. The anhydrous substance readily absorbs water forming pentahydrate and octahydrate.
Praseodymium(III) bromide is a crystalline compound of one praseodymium atom and three bromine atoms.
Praseodymium (III,IV) oxide is the inorganic compound with the formula Pr6O11 that is insoluble in water. It has a cubic fluorite structure. It is the most stable form of praseodymium oxide at ambient temperature and pressure.

Praseodymium(IV) oxide is an inorganic compound with chemical formula PrO2.
Praseodymium(III) fluoride is an inorganic compound with the formula PrF3, being the most stable fluoride of praseodymium.
Praseodymium(III) hydroxide is an inorganic compound with a chemical formula Pr(OH)3.

Praseodymium(III) nitrate is a green-colored chemical compound with the chemical formula Pr(NO3)3. It is very hygroscopic and forms a hexahydrate. It is soluble in polar solvents.