
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. Electrodes are essential parts of batteries that can consist of a variety of materials (chemicals) depending on the type of battery.

A nickel–metal hydride battery is a type of rechargeable battery. The chemical reaction at the positive electrode is similar to that of the nickel–cadmium cell (NiCd), with both using nickel oxide hydroxide (NiOOH). However, the negative electrodes use a hydrogen-absorbing alloy instead of cadmium. NiMH batteries can have two to three times the capacity of NiCd batteries of the same size, with significantly higher energy density, although only about half that of lithium-ion batteries.

A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other commercial rechargeable batteries, Li-ion batteries are characterized by higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991: over the following 30 years, their volumetric energy density increased threefold while their cost dropped tenfold.

A rechargeable battery, storage battery, or secondary cell, is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or primary battery, which is supplied fully charged and discarded after use. It is composed of one or more electrochemical cells. The term "accumulator" is used as it accumulates and stores energy through a reversible electrochemical reaction. Rechargeable batteries are produced in many different shapes and sizes, ranging from button cells to megawatt systems connected to stabilize an electrical distribution network. Several different combinations of electrode materials and electrolytes are used, including lead–acid, zinc–air, nickel–cadmium (NiCd), nickel–metal hydride (NiMH), lithium-ion (Li-ion), lithium iron phosphate (LiFePO4), and lithium-ion polymer.

The AA battery is a standard size single cell cylindrical dry battery. The IEC 60086 system calls the size R6, and ANSI C18 calls it 15. It is named UM-3 by JIS of Japan. Historically, it is known as D14, U12 – later U7, or HP7 in official documentation in the United Kingdom, or a pen cell.

Molten-salt batteries are a class of battery that uses molten salts as an electrolyte and offers both a high energy density and a high power density. Traditional non-rechargeable thermal batteries can be stored in their solid state at room temperature for long periods of time before being activated by heating. Rechargeable liquid-metal batteries are used for industrial power backup, special electric vehiclesand for grid energy storage, to balance out intermittent renewable power sources such as solar panels and wind turbines.

The lithium iron phosphate battery or LFP battery is a type of lithium-ion battery using lithium iron phosphate as the cathode material, and a graphitic carbon electrode with a metallic backing as the anode. Because of their low cost, high safety, low toxicity, long cycle life and other factors, LFP batteries are finding a number of roles in vehicle use, utility-scale stationary applications, and backup power. LFP batteries are cobalt-free. As of September 2022, LFP type battery market share for EVs reached 31%, and of that, 68% were from EV makers Tesla and BYD alone. Chinese manufacturers currently hold a near monopoly of LFP battery type production. With patents having started to expire in 2022 and the increased demand for cheaper EV batteries, LFP type production is expected to rise further and surpass lithium nickel manganese cobalt oxides (NMC) type batteries in 2028.

Lithium iron phosphate or lithium ferro-phosphate (LFP) is an inorganic compound with the formula LiFePO
4. It is a gray, red-grey, brown or black solid that is insoluble in water. The material has attracted attention as a component of lithium iron phosphate batteries, a type of Li-ion battery. This battery chemistry is targeted for use in power tools, electric vehicles, solar energy installations and more recently large grid-scale energy storage.

Lithium cobalt oxide, sometimes called lithium cobaltate or lithium cobaltite, is a chemical compound with formula LiCoO
2. The cobalt atoms are formally in the +3 oxidation state, hence the IUPAC name lithium cobalt(III) oxide.

An electric vehicle battery is a rechargeable battery used to power the electric motors of a battery electric vehicle (BEV) or hybrid electric vehicle (HEV).

An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its negative terminal is the anode. The terminal marked negative is the source of electrons. When a battery is connected to an external electric load, those negatively charged electrons flow through the circuit and reach to the positive terminal, thus cause a redox reaction by attracting positively charged ions, cations. Thus converts high-energy reactants to lower-energy products, and the free-energy difference is delivered to the external circuit as electrical energy. Historically the term "battery" specifically referred to a device composed of multiple cells; however, the usage has evolved to include devices composed of a single cell.

A solid-state battery is an electrical battery that uses a solid electrolyte for ionic conductions between the electrodes, instead of the liquid or gel polymer electrolytes found in conventional batteries. Solid-state batteries theoretically offer much higher energy density than the typical lithium-ion or lithium polymer batteries.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.

Sodium-ion batteries (NIBs, SIBs, or Na-ion batteries) are several types of rechargeable batteries, which use sodium ions (Na+) as their charge carriers. In some cases, its working principle and cell construction are similar to those of lithium-ion battery (LIB) types, but it replaces lithium with sodium as the intercalating ion. Sodium belongs to the same group in the periodic table as lithium and thus has similar chemical properties. However, in some cases, such as aqueous batteries, SIBs can be quite different from LIBs.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and reducing cost.
Khalil Amine is a materials scientist at Argonne National Laboratory, an Argonne distinguished fellow, and group leader of the Battery Technology group. His research team is focused on the development of advanced battery systems for transportation applications. In addition to his Argonne appointment, he is an adjunct professor at Stanford University, Imam Abdulrahman Bin Faisal University, Hong Kong University of Science & Technology, King Abdulaziz University, Hanyang University, and Peking University.
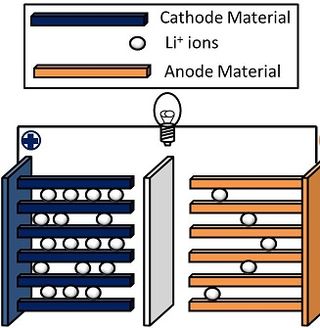
Lithium nickel manganese cobalt oxides (abbreviated NMC, Li-NMC, LNMC, or NCM) are mixed metal oxides of lithium, nickel, manganese and cobalt with the general formula LiNixMnyCo1-x-yO2. These materials are commonly used in lithium-ion batteries for mobile devices and electric vehicles, acting as the positively charged cathode.

Redwood Materials, Inc. is an American company headquartered in Carson City, Nevada. The company aims to recycle lithium-ion batteries and produce battery materials for electromobility and electrical storage systems. Founded in 2017 by J. B. Straubel, Redwood Materials was reported to have a valuation of about $3.7 billion as of July 2021.
A solid-state silicon battery or silicon-anode all-solid-state battery is a type of rechargeable lithium-ion battery consisting of a solid electrolyte, solid cathode, and silicon-based solid anode.

This is a history of the lithium-ion battery.
















