
Silver sulfide is an inorganic compound with the formula Ag
2S. A dense black solid, it is the only sulfide of silver. It is useful as a photosensitizer in photography. It constitutes the tarnish that forms over time on silverware and other silver objects. Silver sulfide is insoluble in most solvents, but is degraded by strong acids. Silver sulfide is a network solid made up of silver and sulfur where the bonds have low ionic character.

Telluric acid, or more accurately orthotelluric acid, is a chemical compound with the formula Te(OH)6, often written as H6TeO6. It is a white crystalline solid made up of octahedral Te(OH)6 molecules which persist in aqueous solution. In the solid state, there are two forms, rhombohedral and monoclinic, and both contain octahedral Te(OH)6 molecules, containing one hexavalent tellurium (Te) atom in the +6 oxidation state, attached to six hydroxyl (–OH) groups, thus, it can be called tellurium(VI) hydroxide. Telluric acid is a weak acid which is dibasic, forming tellurate salts with strong bases and hydrogen tellurate salts with weaker bases or upon hydrolysis of tellurates in water. It is used as tellurium-source in the synthesis of oxidation catalysts.

Bismuth(III) oxide is a compound of bismuth, and a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic) and sphaerobismoite, but it is usually obtained as a by-product of the smelting of copper and lead ores. Dibismuth trioxide is commonly used to produce the "Dragon's eggs" effect in fireworks, as a replacement of red lead.

Carbon tetrabromide, CBr4, also known as tetrabromomethane, is a bromide of carbon. Both names are acceptable under IUPAC nomenclature.

A lattice constant or lattice parameter is one of the physical dimensions and angles that determine the geometry of the unit cells in a crystal lattice, and is proportional to the distance between atoms in the crystal. A simple cubic crystal has only one lattice constant, the distance between atoms, but in general lattices in three dimensions have six lattice constants: the lengths a, b, and c of the three cell edges meeting at a vertex, and the angles α, β, and γ between those edges.

Aluminium sulfide is a chemical compound with the formula Al2S3. This colorless species has an interesting structural chemistry, existing in several forms. The material is sensitive to moisture, hydrolyzing to hydrated aluminium oxides/hydroxides. This can begin when the sulfide is exposed to the atmosphere. The hydrolysis reaction generates gaseous hydrogen sulfide (H2S).
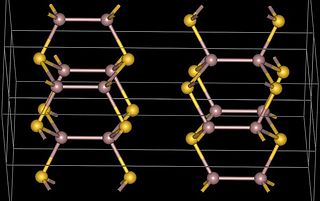
Gallium(II) telluride, GaTe, is a chemical compound of gallium and tellurium. There is research interest in the structure and electronic properties of GaTe because of the possibility that it, or related compounds, may have applications in the electronics industry. Gallium telluride can be made by reacting the elements or by metal organic vapour deposition (MOCVD).

Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4. In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.
The indium chalcogenides include all compounds of indium with the chalcogen elements, oxygen, sulfur, selenium and tellurium. (Polonium is excluded as little is known about its compounds with indium). The best-characterised compounds are the In(III) and In(II) chalcogenides e.g. the sulfides In2S3 and InS.
This group of compounds has attracted a lot of research attention because they include semiconductors, photovoltaics and phase-change materials. In many applications indium chalcogenides are used as the basis of ternary and quaternary compounds such as indium tin oxide, ITO and copper indium gallium selenide, CIGS.
Tellurium iodide is an inorganic compound with the formula TeI. Two forms are known. Their structures differ from the other monohalides of tellurium. There are three subiodides of tellurium, α-TeI, β-TeI, and Te2I, and one tellurium tetraiodide.
Phosphorus selenides are a relatively obscure group of compounds. There have been some studies of the phosphorus - selenium phase diagram and the glassy amorphous phases are reported. The compounds that have been reported are shown below. While some of phosphorus selenides are similar to their sulfide analogues, there are some new forms, molecular P2Se5 and the polymeric catena-[P4Se4]x. There is also some doubt about the existence of molecular P4Se10.
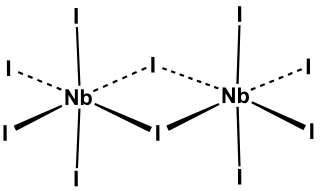
Niobium pentaiodide is the inorganic compound with the formula Nb2I10. Its name comes from the compound's empirical formula, NbI5. It is a diamagnetic, yellow solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two NbI5 units are joined by a pair of iodide bridges. There is no bond between the Nb centres. Niobium(V) chloride, niobium(V) bromide, tantalum(V) chloride, tantalum(V) bromide, and tantalum(V) iodide, all share this structural motif.

Molybdenum(IV) telluride, molybdenum ditelluride or just molybdenum telluride is a compound of molybdenum and tellurium with formula MoTe2, corresponding to a mass percentage of 27.32% molybdenum and 72.68% tellurium.
The telluride bromides are chemical compounds that contain both telluride ions (Te2−) and bromide ions (Br−). They are in the class of mixed anion compounds or chalcogenide halides.
The telluride iodides are chemical compounds that contain both telluride ions (Te2−) and iodide ions (I−). They are in the class of mixed anion compounds or chalcogenide halides.

Cesium sulfide is an inorganic salt with a chemical formula Cs2S. It is a strong alkali in aqueous solution. In the air, cesium sulfide emits rotten egg smelling hydrogen sulfide.

Indium(III) iodide or indium triiodide is a chemical compound of indium and iodine with the formula InI3.
Protactinium(V) bromide is an inorganic compound. It is a halide of protactinium, consisting of protactinium and bromine. It is radioactive and has a chemical formula of PaBr5, which is a red crystal of the monoclinic crystal system.

Rubidium selenide is an inorganic compound composed of selenium and rubidium. It is a selenide with a chemical formula of Rb2Se. Rubidium selenide is used together with caesium selenide in photovoltaic cells.
Neptunium(III) bromide is a bromide of neptunium, with the chemical formula of NpBr3.












