
In chemistry, a polytelluride usually refers to anions of the formula (Ten)2-. Many main group and transition metals form complexes with polytelluride anions. [1]

In chemistry, a polytelluride usually refers to anions of the formula (Ten)2-. Many main group and transition metals form complexes with polytelluride anions. [1]
Conceptually, polytellurides are derived from polytelluranes H2Ten, but such neutral species are not known (even H2Te is labile). Instead, analogous to the preparation of many Zintl ions, polytellurides are produced by reduction of elemental Te with alkali metals. Such reactions can be conducted by heating a mixture of the solids or by dissolving Te metal in amine solvents of alkali metals. Once generated, these alkali metal polytellurides can be converted to lipophilic salts by treatment cryptand ligands or by ion exchange with quat salts.
![Structure of [Cr(Te4)3] . CrTe12 3-.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/a/a0/CrTe12_3-.svg/330px-CrTe12_3-.svg.png)
Salts of polytellurides have often been characterized by X-ray crystallography. Polytelluride salts generally feature open chains, which adopt a zig-zag conformation. In some cases, cyclic structures are observed as in Li2Te7, which features a square-planar Te center bound to two Te-Te-Te chains.
As ligands in coordination complexs, polytellurides are generally bidentate. Complexes of penta-, tetra-, and tritelluride ligands are known. One example is the spirocyclic [Zn(Te4)2]2-. [3]

A coordination complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. A hydroxide attached to a strongly electropositive center may itself ionize, liberating a hydrogen cation (H+), making the parent compound an acid.
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.

In chemistry, a polyoxometalate is a polyatomic ion, usually an anion, that consists of three or more transition metal oxyanions linked together by shared oxygen atoms to form closed 3-dimensional frameworks. The metal atoms are usually group 6 or less commonly group 5 transition metals in their high oxidation states. They are usually colorless or orange, diamagnetic anions. Two broad families are recognized, isopolymetalates, composed of only one kind of metal and oxide, and heteropolymetalates, composed of one metal, oxide, and a main group oxyanion. Many exceptions to these general statements exist.

Chromium(III) chloride (also called chromic chloride) describes any of several compounds of with the formula CrCl3 • xH2O, where x can be 0, 5, and 6. The anhydrous compound with the formula CrCl3 is a violet solid. The most common form of the trichloride is the dark green hexahydrate, CrCl3 • 6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: anions and organic polysulfides. Anions have the general formula S2−
n. These anions are the conjugate bases of the hydrogen polysulfides H2Sn. Organic polysulfides generally have the formulae RSnR, where R = alkyl or aryl.

Cryptands are a family of synthetic bicyclic and polycyclic multidentate ligands for a variety of cations. The Nobel Prize for Chemistry in 1987 was given to Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen for their efforts in discovering and determining uses of cryptands and crown ethers, thus launching the now flourishing field of supramolecular chemistry. The term cryptand implies that this ligand binds substrates in a crypt, interring the guest as in a burial. These molecules are three-dimensional analogues of crown ethers but are more selective and strong as complexes for the guest ions. The resulting complexes are lipophilic.

In organic chemistry, radical anion is a subset of charged free radical species that carry a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .
The term coordination geometry is used in a number of related fields of chemistry and solid state chemistry/physics.

A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.

A coordination polymer is an inorganic or organometallic polymer structure containing metal cation centers linked by ligands. More formally a coordination polymer is a coordination compound with repeating coordination entities extending in 1, 2, or 3 dimensions.
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion/molecule/atom is called a ligand. This number is determined somewhat differently for molecules than for crystals.
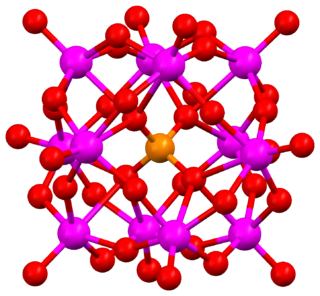
Keggin structure is the best known structural form for heteropoly acids. It is the structural form of α-Keggin anions, which have a general formula of [XM12O40]n−, where X is the heteroatom (most commonly are P5+, Si4+, or B3+), M is the addendum atom (most common are molybdenum and tungsten), and O represents oxygen. The structure self-assembles in acidic aqueous solution and is the most stable structure of polyoxometalate catalysts.

Potassium nonahydridorhenate(VII) is an inorganic compound having the formula K2ReH9. This colourless salt is soluble in water but only poorly soluble in most alcohols. The ReH2−
9 anion is a rare example of a coordination complex bearing only hydride ligands.
Compounds of zinc are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript behavior, they are generally colorless, do not readily engage in redox reactions, and generally adopt symmetrical structures.

Croconic acid or 4,5-dihydroxycyclopentenetrione is a chemical compound with formula C
5H
2O
5 or (C=O)
3(COH)
2. It has a cyclopentene backbone with two hydroxyl groups adjacent to the double bond and three ketone groups on the remaining carbon atoms. It is sensitive to light, soluble in water and ethanol and forms yellow crystals that decompose at 212 °C.
Cyanometallates or cyanometalates are a class of coordination compounds, most often consisting only of cyanide ligands. Most are anions. Cyanide is a highly basic and small ligand, hence it readily saturates the coordination sphere of metal ions. The resulting cyanometallate anions are often used as ligands for building more complex structures called coordination polymers, the best known example of which is Prussian blue, a common dyestuff.
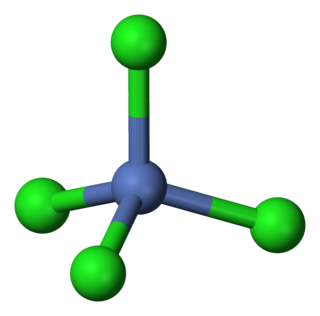
Tetrachloronickelate is the metal complex with the formula [NiCl4]2−. Salts of the complex are available with a variety of cations, but a common one is tetraethylammonium.
The telluride iodides are chemical compounds that contain both telluride ions (Te2−) and iodide ions (I−). They are in the class of mixed anion compounds or chalcogenide halides.

In chemistry, a polyselenide usually refers to anions of the formula (Sen)2-, where Se is the atomic symbol for the element selenium. Many main group and transition metals form complexes with polyselenide anions.