
Aluminium is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating.

Bauxite is a sedimentary rock with a relatively high aluminium content. It is the world's main source of aluminium and gallium. Bauxite consists mostly of the aluminium minerals gibbsite (Al(OH)3), boehmite (γ-AlO(OH)) and diaspore (α-AlO(OH)), mixed with the two iron oxides goethite (FeO(OH)) and haematite (Fe2O3), the aluminium clay mineral kaolinite (Al2Si2O5(OH)4) and small amounts of anatase (TiO2) and ilmenite (FeTiO3 or FeO.TiO2). Bauxite appears dull in luster and is reddish-brown, white, or tan.

Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecules pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid, which crystallizes in needles. There are few commercial applications of acridines; at one time acridine dyes were popular, but they are now relegated to niche applications, such as with acridine orange. The name is a reference to the acrid odour and acrid skin-irritating effect of the compound.
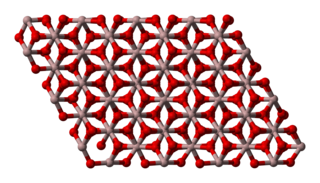
Aluminium oxide (or Aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is significant in its use to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both are colourless crystals, but samples are often contaminated with iron(III) chloride, giving a yellow color.
Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.
The enzyme dihydroxy-acid dehydratase (EC 4.2.1.9) catalyzes the chemical reaction
A dihydroxyanthraquinone is any of several isomeric organic compounds with formula C
14H
8O
4, formally derived from 9,10-anthraquinone by replacing two hydrogen atoms by hydroxyl groups. Dihyroxyantraquinones have been studied since the early 1900s, and include some compounds of historical and current importance. The isomers differ in the position of the hydroxyl groups, and of the carbonyl oxygens (=O) of the underlying anthraquinone.
A dihydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of naphthoquinone through replacement of two hydrogen atoms (H) by hydroxyl groups (OH).
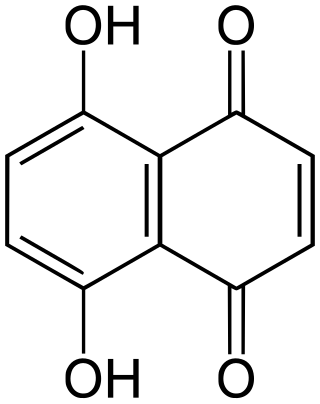
Naphthazarin, often called 5,8-dihydroxy-1,4-naphthoquinone or 5,8-dihydroxy-1,4-naphthalenedione (IUPAC), is a naturally occurring organic compound with formula C
10H
6O
4, formally derived from 1,4-naphthoquinone through replacement of two hydrogen atoms by hydroxyl (OH) groups. It is thus one of many dihydroxynaphthoquinone structural isomers.
A hydroxybenzoquinone is any of several organic compounds that can be viewed as derivatives of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
2,5-Dihydroxy-1,4-benzoquinone or 2,5-dihydroxy-para-benzoquinone is an organic compound with formula C
6H
4O
4, formally derived from 1,4-benzoquinone by replacing two hydrogen atoms with hydroxyl (OH) groups. It is one of seven dihydroxybenzoquinone isomers. It is a yellow solid with planar molecules that exhibits ferroelectric properties.
Isoleucine N-monooxygenase (EC 1.14.13.117, CYP79D3, CYP79D4) is an enzyme with systematic name L-isoleucine,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
Valine N-monooxygenase (EC 1.14.13.118, CYP79D1, CYP79D2) is an enzyme with systematic name L-valine,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
Phenylalanine N-monooxygenase (EC 1.14.14.40, phenylalanine N-hydroxylase, CYP79A2) is an enzyme with systematic name L-phenylalanine,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
Tryptophan N-monooxygenase (EC 1.14.13.125, tryptophan N-hydroxylase, CYP79B1, CYP79B2, CYP79B3) is an enzyme with systematic name L-tryptophan,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction

Zearalanone (ZAN) is a semi-synthetic mycoestrogen that is a derivative of zearalenone (ZEN). Zearalanone is extracted from medical herbs and edible herbs alone with other substance called aflatoxins in the same time by a specific immunoaffinity column. Zearalanone has six analog. They are ZEN, zearalanone, α-zeralanol, β-zeralanol, α-zearalenol, and β-zearalenol.

Homoisoflavonoids (3-benzylidenechroman-4-ones) are a type of phenolic compounds occurring naturally in plants.

Aluminium monoacetate, also known as dibasic aluminium acetate, and formally named dihydroxy aluminium acetate, is a salt of aluminium with acetic acid. It has the formula Al(OH)2(CH3COO), with aluminium in an oxidation state of +3, and appears under standard conditions as a white solid powder.

Cannabitriol ((+)-CBT, (S,S)-9,10-Dihydroxy-Δ6a(10a)-THC) is a phytocannabinoid first isolated in 1966, an oxidation product of tetrahydrocannabinol which has been identified both as a trace component of cannabis and as a metabolite in cannabis users. Its pharmacology has been little studied, though it has been found to act as an antiestrogen and aromatase inhibitor.









