Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes (alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.

Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2(CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic red-brown solids. The soluble trihydrated (n = 3) salt is the usual compound of commerce. It is widely used to prepare compounds used in homogeneous catalysis.
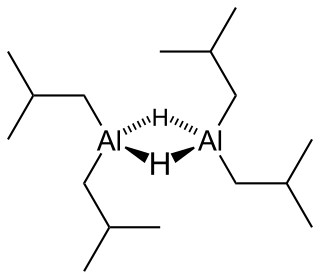
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH) is a reducing agent with the formula (i-Bu2AlH)2, where i-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound is a reagent in organic synthesis.

Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst.
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula C8H12, specifically [−(CH2)2−CH=CH−]2.

Aluminium iodide is a chemical compound containing aluminium and iodine. Invariably, the name refers to a compound of the composition AlI
3, formed by the reaction of aluminium and iodine or the action of HI on Al metal. The hexahydrate is obtained from a reaction between metallic aluminum or aluminum hydroxide with hydrogen iodide or hydroiodic acid. Like the related chloride and bromide, AlI
3 is a strong Lewis acid and will absorb water from the atmosphere. It is employed as a reagent for the scission of certain kinds of C-O and N-O bonds. It cleaves aryl ethers and deoxygenates epoxides.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.

Allylpalladium(II) chloride dimer (APC) is a chemical compound with the formula [(η3-C3H5)PdCl]2. This yellow air-stable compound is an important catalyst used in organic synthesis. It is one of the most widely used transition metal allyl complexes.

Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer triisobutylaluminium, and the titanium-aluminium compound called Tebbe's reagent. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity of the three-coordinated species. Industrially, these compounds are mainly used for the production of polyolefins.
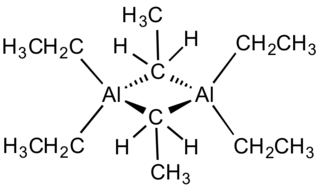
Triethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name the compound has the formula Al2(C2H5)6 (abbreviated as Al2Et6 or TEA). This colorless liquid is pyrophoric. It is an industrially important compound, closely related to trimethylaluminium.

Diethylaluminium cyanide is the organoaluminium compound with formula ( 2AlCN)n. This colorless compound is usually handled as a solution in toluene. It is a reagent for the hydrocyanation of α,β-unsaturated ketones.

Ethylaluminium sesquichloride, also called EASC, is an industrially important organoaluminium compound used primarily as a precursor to triethylaluminium and as a catalyst component in Ziegler–Natta type systems for olefin and diene polymerizations. Other applications include use in alkylation reactions and as a catalyst component in linear oligomerization and cyclization of unsaturated hydrocarbons. EASC is a colourless liquid, spontaneously combustible in air and reacts violently when in contact with water and many other compounds.

Triisobutylaluminium (TiBA) is an organoaluminium compound with the formula Al(CH2CH(CH3)2)3. This colorless pyrophoric liquid is mainly used to make linear primary alcohols and α-olefins.
In organic chemistry, the Ziegler process is a method for producing fatty alcohols from ethylene using an organoaluminium compound. The reaction produces linear primary alcohols with an even numbered carbon chain. The process uses an aluminum compound to oligomerize ethylene and allow the resulting alkyl group to be oxygenated. The usually targeted products are fatty alcohols, which are otherwise derived from natural fats and oils. Fatty alcohols are used in food and chemical processing. They are useful due to their amphipathic nature. The synthesis route is named after Karl Ziegler, who described the process in 1955.

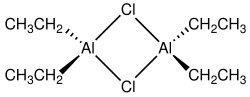
Dimethylaluminium chloride is an organoaluminium compound with the chemical formula [(CH3)2AlCl]2. It behaves similarly to diethylaluminium chloride but is more expensive. Hence, it is less commonly used.

Main group organometallic chemistry concerns the preparation and properties of main-group elements directly bonded to carbon. The inventory is large. The compounds exhibit a wide range of properties, including ones that are water-stable and others that are pyrophoric. Many are very useful themselves, as chemical reagents, or as catalysts.