Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

An oxide is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. Metal oxides thus typically contain an anion of oxygen in the oxidation state of −2. Most of the Earth's crust consists of solid oxides, the result of elements being oxidized by the oxygen in air or in water. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion. Certain elements can form multiple oxides, differing in the amounts of the element combining with the oxygen. Examples are carbon, iron, nitrogen (see nitrogen oxide), silicon, titanium, lithium, and aluminium. In such cases the oxides are distinguished by specifying the numbers of atoms involved, as in carbon monoxide and carbon dioxide, or by specifying the element's oxidation number, as in iron(II) oxide and iron(III) oxide.
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation H3O+, the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton (a positive hydrogen ion, H+) to the surrounding water molecules (H2O). In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous H+ and conjugate base. Three main structures for the aqueous proton have garnered experimental support: The Eigen cation, which is a tetrahydrate, H3O+(H2O)3; the Zundel cation, which is a symmetric dihydrate, H+(H2O)2; and the Stoyanov cation, an expanded Zundel cation, which is a hexahydrate: H+(H2O)2(H2O)4. Spectroscopic evidence from well-defined IR spectra overwhelmingly supports the Stoyanov cation as the predominant form. For this reason, it has been suggested that wherever possible, the symbol H+(aq) should be used instead of the hydronium ion.
Astrochemistry is the study of the abundance and reactions of molecules in the Universe, and their interaction with radiation. The discipline is an overlap of astronomy and chemistry. The word "astrochemistry" may be applied to both the Solar System and the interstellar medium. The study of the abundance of elements and isotope ratios in Solar System objects, such as meteorites, is also called cosmochemistry, while the study of interstellar atoms and molecules and their interaction with radiation is sometimes called molecular astrophysics. The formation, atomic and chemical composition, evolution and fate of molecular gas clouds is of special interest, because it is from these clouds that solar systems form.

The hydroxyl radical is the diatomic molecule •
OH. The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments.

Aluminium chloride (AlCl3), also known as aluminium trichloride, describe compounds with the formula AlCl3(H2O)n (n = 0 or 6). They consist of aluminium and chlorine atoms in a 1:3 ratio, and one form also contains six waters of hydration. Both are white solids, but samples are often contaminated with iron(III) chloride, giving a yellow color.

The trihydrogen cation or protonated molecular hydrogen is a cation with formula H+
3, consisting of three hydrogen nuclei (protons) sharing two electrons.
Aluminium monofluoride, also known as fluoridoaluminium, is the chemical compound with the formula AlF. This elusive species is formed by the reaction between aluminium trifluoride and metallic aluminium at elevated temperatures but quickly reverts to the reactants when cooled. Clusters derived from related aluminium(I) halides can be stabilized using specialized ligands.
The ethynyl radical is an organic compound with the chemical formula C≡CH. It is a simple molecule that does not occur naturally on Earth but is abundant in the interstellar medium. It was first observed by electron spin resonance isolated in a solid argon matrix at liquid helium temperatures in 1963 by Cochran and coworkers at the Johns Hopkins Applied Physics Laboratory. It was first observed in the gas phase by Tucker and coworkers in November 1973 toward the Orion Nebula, using the NRAO 11-meter radio telescope. It has since been detected in a large variety of interstellar environments, including dense molecular clouds, bok globules, star forming regions, the shells around carbon-rich evolved stars, and even in other galaxies.
Interstellar formaldehyde (a topic relevant to astrochemistry) was first discovered in 1969 by L. Snyder et al. using the National Radio Astronomy Observatory. Formaldehyde (H2CO) was detected by means of the 111 - 110 ground state rotational transition at 4830 MHz. On 11 August 2014, astronomers released studies, using the Atacama Large Millimeter/Submillimeter Array (ALMA) for the first time, that detailed the distribution of HCN, HNC, H2CO, and dust inside the comae of comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON).
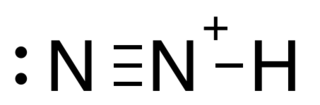
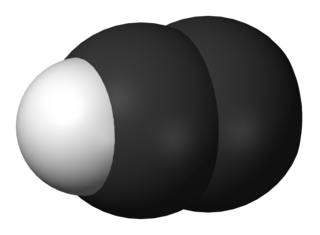
Diazenylium is the chemical N2H+, an inorganic cation that was one of the first ions to be observed in interstellar clouds. Since then, it has been observed for in several different types of interstellar environments, observations that have several different scientific uses. It gives astronomers information about the fractional ionization of gas clouds, the chemistry that happens within those clouds, and it is often used as a tracer for molecules that are not as easily detected (such as N2). Its 1–0 rotational transition occurs at 93.174 GHz, a region of the spectrum where Earth's atmosphere is transparent and it has a significant optical depth in both cold and warm clouds so it is relatively easy to observe with ground-based observatories. The results of N2H+ observations can be used not only for determining the chemistry of interstellar clouds, but also for mapping the density and velocity profiles of these clouds.

HCNH+, also known as protonated hydrogen cyanide, is a molecular ion of astrophysical interest. It also exists in the condensed state when formed by superacids.

Chromium(I) hydride, systematically named chromium hydride, is an inorganic compound with the chemical formula (CrH)
n. It occurs naturally in some kinds of stars where it has been detected by its spectrum. However, molecular chromium(I) hydride with the formula CrH has been isolated in solid gas matrices. The molecular hydride is very reactive. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.

Iron(I) hydride, systematically named iron hydride and poly(hydridoiron) is a solid inorganic compound with the chemical formula (FeH)
n. It is both thermodynamically and kinetically unstable toward decomposition at ambient temperature, and as such, little is known about its bulk properties.
Indium(III) hydroxide is the chemical compound with the formula In(OH)3. Its prime use is as a precursor to indium(III) oxide, In2O3. It is sometimes found as the rare mineral dzhalindite.

Calcium monohydride is a molecule composed of calcium and hydrogen with formula CaH. It can be found in stars as a gas formed when calcium atoms are present with hydrogen atoms.

Magnesium monohydride is a molecular gas with formula MgH that exists at high temperatures, such as the atmospheres of the Sun and stars. It was originally known as magnesium hydride, although that name is now more commonly used when referring to the similar chemical magnesium dihydride.
Tricarbon monosulfide (C3S) or tricarbon sulfur is a reactive molecular substance found in space. Tricarbon monosulfide is a heterocumulene or thiocumulene, consisting of a straight chain of three carbon atoms and a terminal sulfur atom.

Phosphorus monoxide is an unstable radical inorganic compound with molecular formula PO.