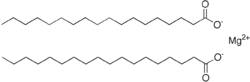
 | |
| Names | |
|---|---|
| IUPAC name Magnesium octadecanoate | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.008.320 |
| E number | E572 (acidity regulators, ...) |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| Mg(C 18H 35O 2) 2 | |
| Molar mass | 591.27 g/mol |
| Appearance | light white powder |
| Odor | slight |
| Density | 1.026 g/cm3 |
| Melting point | 88.5 °C (191.3 °F; 361.6 K) |
| 0.003 g/100 mL (15 °C) 0.004 g/100 mL (25 °C) 0.008 g/100 mL (50 °C) | |
| Solubility | negligible in ether and alcohol slightly soluble in benzene |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 250 °C (482 °F; 523 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | > 1000 mg/kg (oral, rat) |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Magnesium stearate is a metal-organic compound, a salt of magnesium and stearic acid with the idealized chemical formula (C17H35CO2)2Mg. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid. Magnesium stearate is a white, water-insoluble powder. Its applications exploit its softness, insolubility in many solvents, and low toxicity. It is used as a release agent and as a component or lubricant in the production of pharmaceuticals and cosmetics. [1]
