Related Research Articles

Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.

Copper(II) chloride, also known as cupric chloride, is an inorganic compound with the chemical formula CuCl2. The monoclinic yellowish-brown anhydrous form slowly absorbs moisture to form the orthorhombic blue-green dihydrate CuCl2·2H2O, with two water molecules of hydration. It is industrially produced for use as a co-catalyst in the Wacker process.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.
Molybdenum trioxide describes a family of inorganic compounds with the formula MoO3(H2O)n where n = 0, 1, 2. The anhydrous compound is produced on the largest scale of any molybdenum compound since it is the main intermediate produced when molybdenum ores are purified. The anhydrous oxide is a precursor to molybdenum metal, an important alloying agent. It is also an important industrial catalyst. It is a yellow solid, although impure samples can appear blue or green.

Tin(II) chloride, also known as stannous chloride, is a white crystalline solid with the formula SnCl2. It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot. SnCl2 is widely used as a reducing agent (in acid solution), and in electrolytic baths for tin-plating. Tin(II) chloride should not be confused with the other chloride of tin; tin(IV) chloride or stannic chloride (SnCl4).

Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques.

Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid that transitions to white with decreasing crystal size. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten salts. Partly because Li and F are both light elements, and partly because F2 is highly reactive, formation of LiF from the elements releases one of the highest energies per mass of reactants, second only to that of BeO.

Iron(III) phosphate, also ferric phosphate, is the inorganic compound with the formula FePO4. Four polymorphs of anhydrous FePO4 are known. Additionally two polymorphs of the dihydrate FePO4·(H2O)2 are known. These materials have attracted much interest as potential cathode materials in batteries.
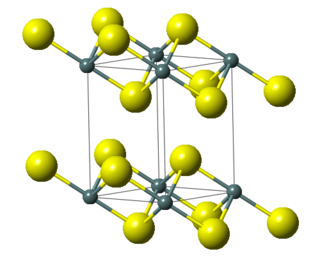
Tin(IV) sulfide is a compound with the formula Sn S
2. The compound crystallizes in the cadmium iodide motif, with the Sn(IV) situated in "octahedral holes' defined by six sulfide centers. It occurs naturally as the rare mineral berndtite. It is useful as semiconductor material with band gap 2.2 eV.

Cadmium acetate is the chemical compound with the formula Cd(O2CCH3)2(H2O)2. The compound is marketed both as the anhydrous form and as a dihydrate, both of which are white or colorless. Only the dihydrate has been verified by X-ray crystallography.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.

Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.
Nickel compounds are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.
The phosphidosilicates or phosphosilicides are inorganic compounds containing silicon bonded to phosphorus and one or more other kinds of elements. In the phosphosilicates each silicon atom is surrounded by four phosphorus atoms in a tetrahedron. The triphosphosilicates have a SiP3 unit, that can be a planar triangle like carbonate CO3. The phosphorus atoms can be shared to form different patterns e.g. [Si2P6]10− which forms pairs, and [Si3P7]3− which contains two-dimensional double layer sheets. [SiP4]8− with isolated tetrahedra, and [SiP2]2− with a three dimensional network with shared tetrahedron corners. SiP clusters can be joined, not only by sharing a P atom, but also by way of a P-P bond. This does not happen with nitridosilicates or plain silicates.
A chloride nitride is a mixed anion compound containing both chloride (Cl−) and nitride ions (N3−). Another name is metallochloronitrides. They are a subclass of halide nitrides or pnictide halides.
Lithium telluride (Li2Te) is an inorganic compound of lithium and tellurium. Along with LiTe3, it is one of the two intermediate solid phases in the lithium-tellurium system. It can be prepared by directly reacting lithium and tellurium in a beryllium oxide crucible at 950°C.
Lithium phosphide is an inorganic compound of lithium and phosphorus with the chemical formula Li3P. This dark colored compound is formally the lithium salt of phosphine, consisting of lithium cations Li+ and phosphide anions P3−. It is hazardous to handle because of its high reactivity toward air.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.
Lithium hexafluorotitanate is an inorganic compound of lithium, fluorine, and titanium with the chemical formula Li2TiF6.
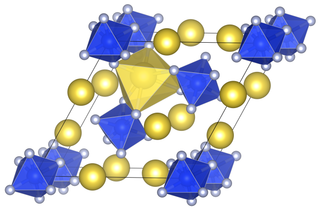
Lithium hexafluorosilicate is an inorganic chemical compound with the chemical formula Li2SiF6.
References
- ↑ "Lithium Hexafluorostannate(IV)". American Elements . Retrieved 23 June 2024.
- ↑ "Lithium (CAS Number 17029-16-2) : Strem Product Catalog". strem.com. Retrieved 23 June 2024.
- ↑ Wilde, W.; Kolditz, L. (November 1987). "Über das thermische Verhalten einiger Lithium‐und Natriumhexafluorometallate(IV)". Zeitschrift für anorganische und allgemeine Chemie . 554 (11): 205–216. doi:10.1002/zaac.19875541127 . Retrieved 25 June 2024.
- ↑ Hebecker, Christoph; Hoppe, Rudolf (1 January 1966). "Neue Untersuchungen an komplexen Fluoriden von Zinn und Blei [1]". Naturwissenschaften (in German). 53 (4): 106–106. doi:10.1007/BF00601472. ISSN 1432-1904 . Retrieved 25 June 2024.
- ↑ Donnay, Joseph Désiré Hubert (1973). Crystal Data: Inorganic compounds. National Bureau of Standards. p. M-163. Retrieved 23 June 2024.
- ↑ Haynes, William M. (9 June 2015). CRC Handbook of Chemistry and Physics, 96th Edition. CRC Press. p. 4-72. ISBN 978-1-4822-6097-7 . Retrieved 23 June 2024.
- ↑ Marseglia, E. A.; Brown, I. D. (15 June 1973). "Lithium hexafluorotitanate dihydrate and lithium hexafluorostannate dihydrate". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 29 (6): 1352–1354. doi:10.1107/S0567740873004498. ISSN 0567-7408 . Retrieved 23 June 2024.