
Caesium is a chemical element; it has symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of 28.5 °C, which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at −116 °C (−177 °F). It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. It has the largest atomic radius of all elements whose radii have been measured or calculated, at about 260 picometers.

The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych.

Caesium hydroxide is a strong base containing the highly reactive alkali metal caesium, much like the other alkali metal hydroxides such as sodium hydroxide and potassium hydroxide. It is the strongest of the five alkali metal hydroxides. Fused Caesium hydroxide dissolves glass by attacking silica framework and it has applications in bringing glass samples into a solution for analytical purposes in commercial glass industry and defense waste processing facility. The melting process is carried out in a nickel or zirconium crucible. Caesium hydroxide fusion at 750°C produces complete dissolution of glass pellets.
This is a list of common chemical compounds with chemical formulae and CAS numbers, indexed by formula. This complements alternative listing at list of inorganic compounds. There is no complete list of chemical compounds since by nature the list would be infinite.

Caesium chloride or cesium chloride is the inorganic compound with the formula CsCl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chloride ions. Caesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Caesium chloride occurs naturally as impurities in carnallite, sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite.

Caesium-137, cesium-137 (US), or radiocaesium, is a radioactive isotope of caesium that is formed as one of the more common fission products by the nuclear fission of uranium-235 and other fissionable isotopes in nuclear reactors and nuclear weapons. Trace quantities also originate from spontaneous fission of uranium-238. It is among the most problematic of the short-to-medium-lifetime fission products. Caesium-137 has a relatively low boiling point of 671 °C (1,240 °F) and easily becomes volatile when released suddenly at high temperature, as in the case of the Chernobyl nuclear accident and with atomic explosions, and can travel very long distances in the air. After being deposited onto the soil as radioactive fallout, it moves and spreads easily in the environment because of the high water solubility of caesium's most common chemical compounds, which are salts. Caesium-137 was discovered by Glenn T. Seaborg and Margaret Melhase.
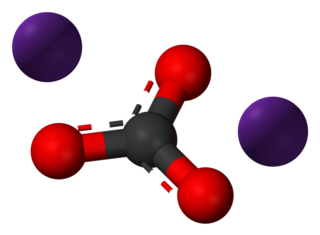
Caesium carbonate or cesium carbonate is a chemical compound with the chemical formula Cs2CO3. It is white crystalline solid. Caesium carbonate has a high solubility in polar solvents such as water, ethanol and DMF. Its solubility is higher in organic solvents compared to other carbonates like potassium carbonate and sodium carbonate, although it remains quite insoluble in other organic solvents such as toluene, p-xylene, and chlorobenzene. This compound is used in organic synthesis as a base. It also appears to have applications in energy conversion.

Caesium bromide or cesium bromide is an ionic compound of caesium and bromine with the chemical formula CsBr. It is a white or transparent solid with melting point at 636 °C that readily dissolves in water. Its bulk crystals have the cubic CsCl structure, but the structure changes to the rocksalt type in nanometer-thin film grown on mica, LiF, KBr or NaCl substrates.

Caesium bicarbonate or cesium bicarbonate is a chemical compound with the chemical formula CsHCO3. It can be produced through the following reaction:

Ethanesulfonic acid (esylic acid) is a sulfonic acid with the chemical formula CH3CH2SO3H. The conjugate base is known as ethanesulfonate or, when used in pharmaceutical formulations, as esilate. It is a colorless liquid.

SouthState Bank, based in Winter Haven, Florida, is an American bank based in Florida and a subsidiary of SouthState Corporation, a bank holding company. As of December 31, 2018, the company had 168 branches in South Carolina, North Carolina, Georgia, Florida, Alabama and Virginia.

Caesium oxalate, or dicesium oxalate, or cesium oxalate is a chemical compound with the chemical formula Cs2C2O4. It is a caesium salt of oxalic acid. It consists of caesium cations Cs+ and oxalate anions C2O2−4.

Cesium sulfide is an inorganic salt with a chemical formula Cs2S. It is a strong alkali in aqueous solution. In the air, cesium sulfide emits rotten egg smelling hydrogen sulfide.

Caesium peroxide or cesium peroxide is an inorganic compound of caesium and oxygen with the chemical formula Cs2O2. It can be formed from caesium metal by adding a stoichiometric amount in ammonia solution, or oxidizing the solid metal directly.

Caesium ozonide is an oxygen-rich chemical compound of caesium, with the chemical formula CsO3. It consists of caesium cations Cs+ and ozonide anions O−3. It can be formed by reacting ozone with caesium superoxide:
Lithium hexafluoroarsenate is an inorganic chemical compound with the chemical formula LiAsF6.
Caesium telluride or Caesium telluridocaesium is an inorganic salt with a chemical formula Cs2Te. Caesium telluride is used to make photo cathodes.
Sodium hexafluoroarsenate is an inorganic chemical compound with the chemical formula NaAsF6.
Potassium hexafluoroantimonate is an inorganic chemical compound with the chemical formula KSbF6.

Barium hexafluorosilicate is an inorganic chemical compound with the chemical formula BaSiF6.

















