 | |
| Names | |
|---|---|
| Other names Cesium hexafluorocuprate; Dicesium hexafluorocuprate | |
| Identifiers | |
3D model (JSmol) | |
| |
| |
| Properties | |
| Cs2CuF6 | |
| Molar mass | 443.35 g/mol |
| Appearance | Red orange solid [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
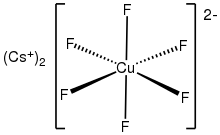
Caesium hexafluorocuprate is the inorganic compound with the chemical formula Cs2CuF6. It is a red solid that degrades upon contact with water. It was first prepared by heating CsCuCl
3 and caesium fluoride at 410 °C under 350 atmospheres of fluorine: [2]
- 2 CsCuCl3 + 2 CsF + 5 F2 → 2 Cs2CuF6 + 3 Cl2
The anion [CuF6]2- is a rare example of a copper(IV) complex. In terms of its electronic structure, the anion has a low-spin d7 configuration. It is thus susceptible to Jahn-Teller distortion. [3]