
High-temperature superconductors are defined as materials with critical temperature above 77 K, the boiling point of liquid nitrogen. They are only "high-temperature" relative to previously known superconductors, which function at even colder temperatures, close to absolute zero. The "high temperatures" are still far below ambient, and therefore require cooling. The first breakthrough of high-temperature superconductor was discovered in 1986 by IBM researchers Georg Bednorz and K. Alex Müller. Although the critical temperature is around 35.1 K, this new type of superconductor was readily modified by Ching-Wu Chu to make the first high-temperature superconductor with critical temperature 93 K. Bednorz and Müller were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-Tc materials are type-II superconductors.
In chemistry, diamondoids are generalizations of the carbon cage molecule known as adamantane (C10H16), the smallest unit cage structure of the diamond crystal lattice. Diamondoids also known as nanodiamonds or condensed adamantanes may include one or more cages (adamantane, diamantane, triamantane, and higher polymantanes) as well as numerous isomeric and structural variants of adamantanes and polymantanes. These diamondoids occur naturally in petroleum deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule. These species are of interest as molecular approximations of the diamond cubic framework, terminated with C−H bonds.

A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.

Quantum dots (QDs) or semiconductor nanocrystals are semiconductor particles a few nanometres in size with optical and electronic properties that differ from those of larger particles via quantum mechanical effects. They are a central topic in nanotechnology and materials science. When a quantum dot is illuminated by UV light, an electron in the quantum dot can be excited to a state of higher energy. In the case of a semiconducting quantum dot, this process corresponds to the transition of an electron from the valence band to the conductance band. The excited electron can drop back into the valence band releasing its energy as light. This light emission (photoluminescence) is illustrated in the figure on the right. The color of that light depends on the energy difference between the conductance band and the valence band, or the transition between discrete energy states when the band structure is no longer well-defined in QDs.

Lead(II) iodide is a chemical compound with the formula PbI
2. At room temperature, it is a bright yellow odorless crystalline solid, that becomes orange and red when heated. It was formerly called plumbous iodide.

A quantum dot solar cell (QDSC) is a solar cell design that uses quantum dots as the captivating photovoltaic material. It attempts to replace bulk materials such as silicon, copper indium gallium selenide (CIGS) or cadmium telluride (CdTe). Quantum dots have bandgaps that are adjustable across a wide range of energy levels by changing their size. In bulk materials, the bandgap is fixed by the choice of material(s). This property makes quantum dots attractive for multi-junction solar cells, where a variety of materials are used to improve efficiency by harvesting multiple portions of the solar spectrum.
Resistive random-access memory is a type of non-volatile (NV) random-access (RAM) computer memory that works by changing the resistance across a dielectric solid-state material, often referred to as a memristor. One major advantage of ReRAM over other NVRAM technologies is the ability to scale below 10 nm.
Perfluorodecalin is a fluorocarbon, a derivative of decalin in which all of the hydrogen atoms are replaced by fluorine atoms. It is chemically and biologically inert and stable up to 400 °C. Several applications make use of its ability to dissolve gases.

Bismuth tribromide is an inorganic compound of bismuth and bromine with the chemical formula BiBr3.

Ruddlesden-Popper (RP) phases are a type of perovskite structure that consists of two-dimensional perovskite-like slabs interleaved with cations. The general formula of an RP phase is An+1BnX3n+1, where A and B are cations, X is an anion, and n is the number of octahedral layers in the perovskite-like stack. Generally, it has a phase structure that results from the intergrowth of perovskite-type and NaCl-type structures.

A perovskite solar cell (PSC) is a type of solar cell that includes a perovskite-structured compound, most commonly a hybrid organic–inorganic lead or tin halide-based material as the light-harvesting active layer. Perovskite materials, such as methylammonium lead halides and all-inorganic cesium lead halide, are cheap to produce and simple to manufacture.

Lead bismuthate is a chemical compound with the formula Pb(BiO3)2. It has only been discovered in recent years in the laboratory as it is not naturally occurring. Lead bismuthate forms a pentavalent structure, significantly different from the regular ionic interactions of sodium bismuthate, but similar to that of strontium bismuthate. In the structure, six oxygen atoms are coordinated octahedrally to both the bismuth and lead atoms. The bismuth and oxygen atoms form negatively charged layers by creating repeating octahedral geometries. The positively charged lead atoms are then disbursed within the layers, forming a hexagonal unit cell, with a lead atom in each of the corners. The density of the crystal is 9.18 g/cm3. The formula weight is 233.99 g/mol. The volume of the crystal structure unit is 169.26 A3. Lattice parameters (a) is 5.321 angstroms.
Quantum dots (QDs) are semiconductor nanoparticles with a size less than 10 nm. They exhibited size-dependent properties especially in the optical absorption and the photoluminescence (PL). Typically, the fluorescence emission peak of the QDs can be tuned by changing their diameters. So far, QDs were consisted of different group elements such as CdTe, CdSe, CdS in the II-VI category, InP or InAs in the III-V category, CuInS2 or AgInS2 in the I–III–VI2 category, and PbSe/PbS in the IV-VI category. These QDs are promising candidates as fluorescent labels in various biological applications such as bioimaging, biosensing and drug delivery.

Maksym V. Kovalenko is a full professor of inorganic chemistry and the head of the Functional Inorganic Materials group at ETH Zurich. A part of the research activities of the group are conducted at Empa (Dübendorf). He is working in the fields of solid-state chemistry, quantum dots and other nanomaterials, surface chemistry, self-assembly, optical spectroscopy, optoelectronics and energy storage.

Perovskite nanocrystals are a class of semiconductor nanocrystals, which exhibit unique characteristics that separate them from traditional quantum dots. Perovskite nanocrystals have an ABX3 composition where A = cesium, methylammonium (MA), or formamidinium (FA); B = lead or tin; and X = chloride, bromide, or iodide.
Arsenide bromides or bromide arsenides are compounds containing anions composed of bromide (Br−) and arsenide (As3−). They can be considered as mixed anion compounds. They are in the category of pnictidehalides. Related compounds include the arsenide chlorides, arsenide iodides, phosphide bromides, and antimonide bromides.

Caesium enneabromodibismuthate is an inorganic compound with the formula Cs3Bi2Br9. It is one of the coordination complexes formed by caesium, bismuth and bromine. At room temperature, it is trigonal (P3m1) and it undergoes phase transformation to monoclinic phase (C12/c1) when the temperature is below 96 K.
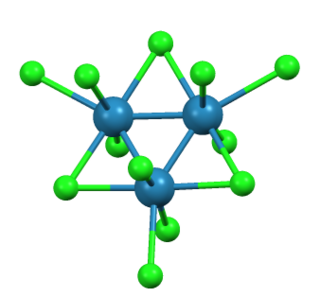
Rhenium(III) bromide is a chemical compound with the formula Re3Br9. It is a black lustrous crystalline solid. This compound reacts with water to form rhenium(IV) oxide and is isostructural with rhenium(III) chloride.
Bromoantimonates are compounds containing anions composed of bromide (Br−) and antimony (Sb). They can be considered as double bromides, metallohalides or halometallates. They are in the category of halopnictates. Related compounds include the bromobismuthates, iodoantimonates, bromophosphates, and bromoarsenates.
Simon Redfern is a mineralogist, geoscientist and academic. He is the Dean of the College of Science, the President's Chair in Earth Sciences, and a professor at Nanyang Technological University (NTU) Singapore as well as an emeritus Professorial Fellow at Jesus College, University of Cambridge.













