 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
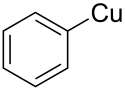
| C6H5Cu | |
| Molar mass | 140.652 g·mol−1 |
| Appearance | Colorless crystals |
| reacts with water | |
| Related compounds | |
Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Phenylcopper is an organometallic chemical compound of copper. [1] Its chemical formula is C 6 H 5 Cu , [2] where copper is in the oxidation state of +1.