
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) molecular ion with the chemical formula NH+4 or [NH4]+. It is formed by the addition of a proton to ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups. Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, human impact in recent years could have an effect on the biological communities that depend on it.

Iridium(III) chloride is the inorganic compound with the formula IrCl3. The anhydrous compound is relatively rare, but the related hydrate is much more commonly encountered. The anhydrous salt has two polymorphs, α and β, which are brown and red colored respectively. More commonly encountered is the hygroscopic dark green trihydrate IrCl3(H2O)3 which is a common starting point for iridium chemistry.

Ammonium hydrosulfide is the chemical compound with the formula [NH4]SH.

Terbium(III,IV) oxide, occasionally called tetraterbium heptaoxide, has the formula Tb4O7, though some texts refer to it as TbO1.75. There is some debate as to whether it is a discrete compound, or simply one phase in an interstitial oxide system. Tb4O7 is one of the main commercial terbium compounds, and the only such product containing at least some Tb(IV) (terbium in the +4 oxidation state), along with the more stable Tb(III). It is produced by heating the metal oxalate, and it is used in the preparation of other terbium compounds. Terbium forms three other major oxides: Tb2O3, TbO2, and Tb6O11.

Chloroplatinic acid (also known as hexachloroplatinic acid) is an inorganic compound with the formula [H3O]2[PtCl6](H2O)x (0 ≤ x ≤ 6). A red solid, it is an important commercial source of platinum, usually as an aqueous solution. Although often written in shorthand as H2PtCl6, it is the hydronium (H3O+) salt of the hexachloroplatinate anion (PtCl2−
6). Hexachloroplatinic acid is highly hygroscopic.

Ytterbium(III) chloride (YbCl3) is an inorganic chemical compound. It reacts with NiCl2 to form a very effective catalyst for the reductive dehalogenation of aryl halides. It is poisonous if injected, and mildly toxic by ingestion. It is an experimental teratogen, known to irritate the skin and eyes.

Thorium(IV) chloride describes a family of inorganic compounds with the formula ThCl4(H2O)n. Both the anhydrous and tetrahydrate (n = 4) forms are known. They are hygroscopic, water-soluble white salts.
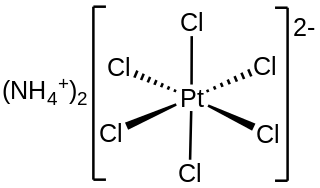
Ammonium hexachloroplatinate, also known as ammonium chloroplatinate, is the inorganic compound with the formula (NH4)2[PtCl6]. It is a rare example of a soluble platinum(IV) salt that is not hygroscopic. It forms intensely yellow solutions in water. In the presence of 1M NH4Cl, its solubility is only 0.0028 g/100 mL.
Ammonium fluorosilicate (also known as ammonium hexafluorosilicate, ammonium fluosilicate or ammonium silicofluoride) has the formula (NH4)2SiF6. It is a toxic chemical, like all salts of fluorosilicic acid. It is made of white crystals, which have at least three polymorphs and appears in nature as rare minerals cryptohalite or bararite.

Sodium hexachloroplatinate(IV), the sodium salt of chloroplatinic acid, is an inorganic compound with the formula Na2[PtCl6], consisting of the sodium cation and the hexachloroplatinate anion. As explained by Cox and Peters, anhydrous sodium hexachloroplatinate, which is yellow, tends to form the orange hexahydrate upon storage in humid air. The latter can be dehydrated upon heating at 110 °C.

Lead tetrachloride, also known as lead(IV) chloride, has the molecular formula PbCl4. It is a yellow, oily liquid which is stable below 0 °C, and decomposes at 50 °C. It has a tetrahedral configuration, with lead as the central atom. The Pb–Cl covalent bonds have been measured to be 247 pm and the bond energy is 243 kJ⋅mol−1.

Ammonium hexachloroiridate(IV) is the inorganic compound with the formula (NH4)2[IrCl6]. This dark red solid is the ammonium salt of the iridium(IV) complex [IrCl6]2−. It is a commercially important iridium compound one of the most common complexes of iridium(IV). A related but ill-defined compound is iridium tetrachloride, which is often used interchangeably.
Iridium tetrachloride is an inorganic compound with the approximate formula IrCl4(H2O)n. It is a water-soluble dark brown amorphous solid. A well defined derivative is ammonium hexachloroiridate ((NH4)2IrCl6). It is used to prepare catalysts, such as the Henbest Catalyst for transfer hydrogenation of cyclohexanones.
Polonium sulfide is an inorganic compound of polonium and sulfur with the chemical formula PoS. The compound is radioactive and forms black crystals.
Lanthanide trichlorides are a family of inorganic compound with the formula LnCl3, where Ln stands for a lanthanide metal. The trichlorides are standard reagents in applied and academic chemistry of the lanthanides. They exist as anhydrous solids and as hydrates.

Ammonium hexachlorotellurate is an inorganic chemical compound with the chemical formula [NH4]2[TeCl6]. It forms yellow octahedral crystals about 0.1 mm (0.0039 in) diameter, decomposes gradually in air. The compound contains the ammonium cations [NH4]+ and hexachlorotellurate(IV) anions [TeCl6]2−.
Lithium hexafluorostannate is an inorganic chemical compound with the chemical formula Li2SnF6.
Ammonium hexafluorostannate is an inorganic chemical compound with the chemical formula (NH4)2SnF6.
Ammonium hexafluoroferrate is an inorganic chemical compound with the chemical formula (NH4)3FeF6.
Ammonium hexafluorovanadate is an inorganic chemical compound with the chemical formula (NH4)3VF6.












