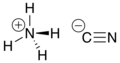Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil.

In chemistry, a cyanide is a chemical compound that contains a C≡N functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Hydrogen cyanide is a chemical compound with the formula HCN and structural formula H−C≡N. It is a highly toxic and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. A solution of hydrogen cyanide in water, represented as HCN, is called hydrocyanic acid. The salts of the cyanide anion are known as cyanides.

Potassium ferrocyanide is the inorganic compound with formula K4[Fe(CN)6]·3H2O. It is the potassium salt of the coordination complex [Fe(CN)6]4−. This salt forms lemon-yellow monoclinic crystals.

Sodium cyanide is a poisonous compound with the formula NaCN. It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its high reactivity toward metals. It is a moderately strong base.

The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups. Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, the human impact in recent years could have an effect on the biological communities that depend on it.

Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion. It is soluble in water and its solution shows some green-yellow fluorescence. It was discovered in 1822 by Leopold Gmelin.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The name of the compound is composed of a base, which includes the carbon of the −C≡N, suffixed with "nitrile", so for example CH3CH2C≡N is called "propionitrile". The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.

Potassium cyanide is a compound with the formula KCN. It is a colorless salt, similar in appearance to sugar, that is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewellery for chemical gilding and buffing. Potassium cyanide is highly toxic, and a dose of 200 to 300 milligrams will kill nearly any human.

Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticides, and in the manufacture of hydrocyanic acid. It has been used as a softener for paper and fiber. It is a solvent for many ionic compounds. It has also been used as a solvent for resins and plasticizers. Some astrobiologists suggest that it may be an alternative to water as the main solvent in other forms of life.
The Wöhler synthesis is the conversion of ammonium cyanate into urea. This chemical reaction was described in 1828 by Friedrich Wöhler. It is often cited as the starting point of modern organic chemistry. Although the Wöhler reaction concerns the conversion of ammonium cyanate, this salt appears only as an (unstable) intermediate. Wöhler demonstrated the reaction in his original publication with different sets of reactants: a combination of cyanic acid and ammonia, a combination of silver cyanate and ammonium chloride, a combination of lead cyanate and ammonia and finally from a combination of mercury cyanate and cyanatic ammonia.

Mercury(II) cyanide, also known as mercuric cyanide, is a poisonous compound of mercury and cyanide. It is an odorless, toxic white powder. It is highly soluble in polar solvents such as water, alcohol, and ammonia; slightly soluble in ether; and insoluble in benzene and other hydrophobic solvents.
The Andrussow process is the dominant industrial process for the production of hydrogen cyanide. It involves the reaction of methane, ammonia, and oxygen. The process is catalyzed by a platinum-rhodium alloy.
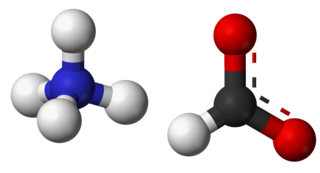
Ammonium formate, NH4HCO2, is the ammonium salt of formic acid. It is a colorless, hygroscopic, crystalline solid.
The BMA process or Degussa process is a chemical process developed by the German chemical company Degussa for the production of hydrogen cyanide from methane and ammonia in presence of a platinum catalyst. Hydrogen cyanide is used in the chemical industry for the production of intermediate chemicals like acrylonitrile, methyl methacrylate, and adiponitrile.

Ammonium thiocyanate is an inorganic compound with the formula [NH4]+[SCN]−. It is an ammonium salt of thiocyanic acid. It consists of ammonium cations [NH4]+ and thiocyanate anions [SCN]−.

Calcium cyanide is the inorganic compound with the formula Ca(CN)2. It is the calcium salt derived from hydrocyanic acid. It is a white solid, although the pure material is rarely encountered. It hydrolyses readily (even in moist air) to release hydrogen cyanide and is very toxic.
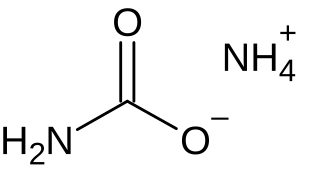
Ammonium carbamate is a chemical compound with the formula [NH4][H2NCO2] consisting of ammonium cation NH+4 and carbamate anion NH2COO−. It is a white solid that is extremely soluble in water, less so in alcohol. Ammonium carbamate can be formed by the reaction of ammonia NH3 with carbon dioxide CO2, and will slowly decompose to those gases at ordinary temperatures and pressures. It is an intermediate in the industrial synthesis of urea (NH2)2CO, an important fertilizer.
Aluminium cyanide is a metallic cyanide with a chemical formula of Al(CN)3. It is a white solid that undergoes hydrolysis to produce aluminium hydroxide and hydrogen cyanide.
Gold(I) cyanide is the inorganic compound with the chemical formula AuCN. It is the binary cyanide of gold(I). It is an odourless, tasteless yellow solid. Wet gold(I) cyanide is unstable to light and will become greenish. Gold(I) cyanide itself is only of academic interest, but its derivative dicyanoaurate is an intermediate in gold cyanidation, the extraction of gold from its ores.