Sodium laureth sulfate (SLES), an accepted contraction of sodium lauryl ether sulfate (SLES), also called sodium alkylethersulfate, is an anionic detergent and surfactant found in many personal care products and for industrial uses. SLES is an inexpensive and very effective foaming agent. SLES, sodium lauryl sulfate (SLS), ammonium lauryl sulfate (ALS), and sodium pareth sulfate are surfactants that are used in many cosmetic products for their cleaning and emulsifying properties. It is derived from palm kernel oil or coconut oil. In herbicides, it is used as a surfactant to improve absorption of the herbicidal chemicals and reduces time the product takes to be rainfast, when enough of the herbicidal agent will be absorbed.
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula CH3(CH2)11OSO3Na and structure H3C−(CH2)11−O−S(=O)2−O−Na+. It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt of the 12-carbon organosulfate. Its hydrocarbon tail combined with a polar "headgroup" give the compound amphiphilic properties that make it useful as a detergent. SDS is also component of mixtures produced from inexpensive coconut and palm oils. SDS is a common component of many domestic cleaning, personal hygiene and cosmetic, pharmaceutical, and food products, as well as of industrial and commercial cleaning and product formulations.

A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more soluble in hard water, because the polar sulfonate is less likely than the polar carboxylate to bind to calcium and other ions found in hard water.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word "surfactant" is a blend of surface-active agent, coined c. 1950. As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt.
In organic chemistry, ethoxylation is a chemical reaction in which ethylene oxide adds to a substrate. It is the most widely practiced alkoxylation, which involves the addition of epoxides to substrates.

In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure [NR4]+, where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.
Fatty alcohols (or long-chain alcohols) are usually high-molecular-weight, straight-chain primary alcohols, but can also range from as few as 4–6 carbons to as many as 22–26, derived from natural fats and oils. The precise chain length varies with the source. Some commercially important fatty alcohols are lauryl, stearyl, and oleyl alcohols. They are colourless oily liquids (for smaller carbon numbers) or waxy solids, although impure samples may appear yellow. Fatty alcohols usually have an even number of carbon atoms and a single alcohol group (–OH) attached to the terminal carbon. Some are unsaturated and some are branched. They are widely used in industry. As with fatty acids, they are often referred to generically by the number of carbon atoms in the molecule, such as "a C12 alcohol", that is an alcohol having 12 carbons, for example dodecanol.
Dodecanol, or lauryl alcohol, is an organic compound produced industrially from palm kernel oil or coconut oil. It is a fatty alcohol. Sulfate esters of lauryl alcohol, especially sodium lauryl sulfate, are very widely used as surfactants. Sodium lauryl sulfate and the related dodecanol derivatives ammonium lauryl sulfate and sodium laureth sulfate are all used in shampoos. Dodecanol is tasteless, colorless, and has a floral odor.
A foaming agent is a material such as a surfactant or a blowing agent that facilitates the formation of foam. A surfactant, when present in small amounts, reduces surface tension of a liquid or increases its colloidal stability by inhibiting coalescence of bubbles. A blowing agent is a gas that forms the gaseous part of the foam.
Sodium myreth sulfate is a mixture of organic compounds with both detergent and surfactant properties. It is found in many personal care products such as soaps, shampoos, and toothpaste. It is an inexpensive and effective foaming agent. Typical of many detergents, sodium myreth sulfate consists of several closely related compounds. Sometimes the number of ethylene glycol ether units (n) is specified in the name as myreth-n sulfate, for example myreth-2 sulfate.

An amphiphile, or amphipath, is a chemical compound possessing both hydrophilic and lipophilic (fat-loving) properties. Such a compound is called amphiphilic or amphipathic. Amphiphilic compounds include surfactants and detergents. The phospholipid amphiphiles are the major structural component of cell membranes.

In chemistry, an amine oxide, also known as an amine N-oxide or simply N-oxide, is a chemical compound that has the chemical formula R3N+−O−. It contains a nitrogen-oxygen coordinate covalent bond with three additional hydrogen and/or substituent-groups attached to nitrogen. Sometimes it is written as R3N→O or, alternatively, as R3N=O.

Cetrimonium bromide, also known with the abbreviation CTAB, is a quaternary ammonium surfactant with a condensed structural formula [(C16H33)N(CH3)3]Br.

Baby shampoo is a hair care product that is used for the removal of oils, dirt, skin particles, dandruff, environmental pollutants and other contaminant particles that gradually build up in hair; specially formulated for use on infants and young children by means of substituting chemicals which are purportedly less irritating to the eyes than those commonly found in regular shampoo.
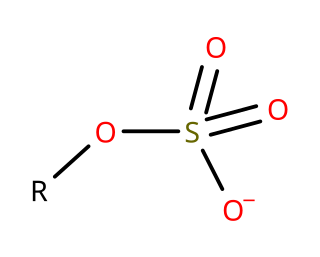
In organosulfur chemistry, organosulfates are a class of organic compounds sharing a common functional group with the structure R−O−SO−3. The SO4 core is a sulfate group and the R group is any organic residue. All organosulfates are formally esters derived from alcohols and sulfuric acid although many are not prepared in this way. Many sulfate esters are used in detergents, and some are useful reagents. Alkyl sulfates consist of a hydrophobic hydrocarbon chain, a polar sulfate group and either a cation or amine to neutralize the sulfate group. Examples include: sodium lauryl sulfate and related potassium and ammonium salts.
A hydrotrope is a compound that solubilizes hydrophobic compounds in aqueous solutions by means other than micellar solubilization. Typically, hydrotropes consist of a hydrophilic part and a hydrophobic part, but the hydrophobic part is generally too small to cause spontaneous self-aggregation. Hydrotropes do not have a critical concentration above which self-aggregation spontaneously starts to occur. Instead, some hydrotropes aggregate in a step-wise self-aggregation process, gradually increasing aggregation size. However, many hydrotropes do not seem to self-aggregate at all, unless a solubilizate has been added. Examples of hydrotropes include urea, tosylate, cumenesulfonate and xylenesulfonate.

Shampoo is a hair care product, typically in the form of a viscous liquid, that is used for cleaning hair. Less commonly, shampoo is available in solid bar format. Shampoo is used by applying it to wet hair, massaging the product into the scalp, and then rinsing it out. Some users may follow a shampooing with the use of hair conditioner.
A soap substitute is a natural or synthetic cleaning product used in place of soap or other detergents, typically to reduce environmental impact or health harms or provide other benefits.
Hair washing without commercial shampoo, sometimes called no poo, includes water-only hair washing or hair washing with non-commercial products, such as baking soda and vinegar. Advocates argue that commercial shampoo is an unnecessary expense and may contain harmful ingredients.
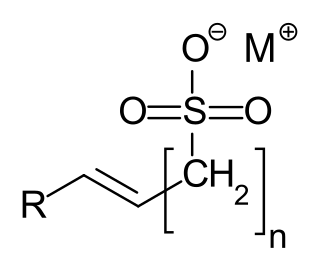
α-Olefin sulfonates are a group of anionic surfactants, which are used as detergents. The compounds contain a - mostly linear, primary - alkyl R and a monovalent cation M, preferably sodium. The most frequently used example of this group of substances is sodium α-olefin sulfonate.










