Sodium azide is an inorganic compound with the formula NaN3. This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance, is highly soluble in water, and is acutely poisonous.

Europium(III) chloride is an inorganic compound with the formula EuCl3. The anhydrous compound is a yellow solid. Being hygroscopic it rapidly absorbs water to form a white crystalline hexahydrate, EuCl3·6H2O, which is colourless. The compound is used in research.

Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.

Ammonium fluoride is the inorganic compound with the formula NH4F. It crystallizes as small colourless prisms, having a sharp saline taste, and is highly soluble in water. Like all fluoride salts, it is moderately toxic in both acute and chronic overdose.

Ammonium persulfate (APS) is the inorganic compound with the formula (NH4)2S2O8. It is a colourless (white) salt that is highly soluble in water, much more so than the related potassium salt. It is a strong oxidizing agent that is used as a catalyst in polymer chemistry, as an etchant, and as a cleaning and bleaching agent.

Ammonium hydrosulfide is the chemical compound with the formula [NH4]SH.
Pentazole is an aromatic molecule consisting of a five-membered ring with all nitrogen atoms, one of which is bonded to a hydrogen atom. It has the molecular formula HN5. Although strictly speaking a homocyclic, inorganic compound, pentazole has historically been classed as the last in a series of heterocyclic azole compounds containing one to five nitrogen atoms. This set contains pyrrole, imidazole, pyrazole, triazoles, tetrazole, and pentazole.

Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This vivid orange, opaque, crystalline explosive is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.

Erbium(III) chloride is a violet solid with the formula ErCl3. It is used in the preparation of erbium metal.

Reinecke's salt is an inorganic compound with the formula NH4[Cr(NCS)4(NH3)2]·H2O. The dark-red crystalline compound is soluble in boiling water, acetone, and ethanol. It can be classified as a metal isothiocyanate complex.

Silver azide is the chemical compound with the formula AgN3. It is a silver(I) salt of hydrazoic acid. It forms a colorless crystals. Like most azides, it is a primary explosive.

Vanadium(III) fluoride is the chemical compound with the formula VF3. This yellow-green, refractory solid is obtained in a two-step procedure from V2O3. Similar to other transition-metal fluorides (such as MnF2), it exhibits magnetic ordering at low temperatures (e.g. V2F6.4H2O orders below 12 K).
Tutton's salts are a family of salts with the formula M2M'(SO4)2(H2O)6 (sulfates) or M2M'(SeO4)2(H2O)6 (selenates). These materials are double salts, which means that they contain two different cations, M+ and M'2+ crystallized in the same regular ionic lattice. The univalent cation can be potassium, rubidium, caesium, ammonium (NH4), deuterated ammonium (ND4) or thallium. Sodium or lithium ions are too small. The divalent cation can be magnesium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc or cadmium. In addition to sulfate and selenate, the divalent anion can be chromate (CrO42−), tetrafluoroberyllate (BeF42−), hydrogenphosphate (HPO42−) or monofluorophosphate (PO3F2−). Tutton's salts crystallize in the monoclinic space group P21/a. The robustness is the result of the complementary hydrogen-bonding between the tetrahedral anions and cations as well their interactions with the metal aquo complex [M(H2O)6]2+.

Potassium azide is the inorganic compound having the formula KN3. It is a white, water-soluble salt. It is used as a reagent in the laboratory.
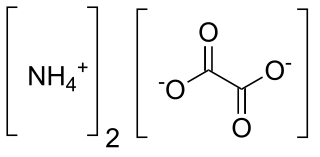
Ammonium oxalate is a chemical compound with the chemical formula [NH4]2C2O4. Its formula is often written as (NH4)2C2O4 or (COONH4)2. It is an ammonium salt of oxalic acid. It consists of ammonium cations ([NH4]+) and oxalate anions (C2O2−4). The structure of ammonium oxalate is ([NH4]+)2[C2O4]2−. Ammonium oxalate sometimes comes as a monohydrate ([NH4]2C2O4·H2O). It is a colorless or white salt under standard conditions and is odorless and non-volatile. It occurs in many plants and vegetables.

Rubidium azide is an inorganic compound with the formula RbN3. It is the rubidium salt of the hydrazoic acid HN3. Like most azides, it is explosive.
Hydrazinium azide or hydrazine azide is a chemical compound with formula H
5N
5 or [N
2H+
5][N−
3]. It is a salt of the hydrazinium cation N
2H+
5 and the azide anion N−
3. It can be seen as a derivative of hydrazine N
2H
4 and hydrazoic acid HN
3. It is an unstable solid.

Caesium azide or cesium azide is an inorganic compound of caesium and nitrogen. It is a salt of azide with the formula CsN3.

Sulfuryl diazide or sulfuryl azide is a chemical compound with the molecular formula SO2(N3)2. It was first described in the 1920s when its reactions with benzene and p-xylene were studied by Theodor Curtius and Karl Friedrich Schmidt. The compound is reported as having "exceedingly explosive, unpredictable properties" and "in many cases very violent explosions occurred without any apparent reason".

Transition metal azide complexes are coordination complexes containing one or more azide (N3−) ligands. In addition to coordination complexes, this article summarizes homoleptic transition metal azides, which are often coordination polymers.