Related Research Articles

Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.
In chemistry, azide is a linear, polyatomic anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.

Sodium azide is an inorganic compound with the formula NaN3. This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance, is highly soluble in water, and is acutely poisonous.

Silver azide is the chemical compound with the formula AgN3. It is a silver(I) salt of hydrazoic acid. It forms a colorless crystals. Like most azides, it is a primary explosive.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Copper(II) azide is a medium density explosive with the molecular formula Cu(N3)2.
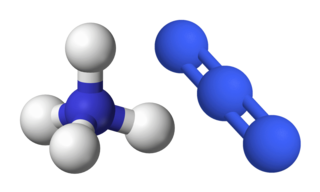
Ammonium azide is the chemical compound with the formula [NH4]N3, being the salt of ammonia and hydrazoic acid. Like other inorganic azides, this colourless crystalline salt is a powerful explosive, although it has a remarkably low sensitivity. [NH4]N3 is physiologically active and inhalation of small amounts causes headaches and palpitations. It was first obtained by Theodor Curtius in 1890, along with other azides.

Potassium azide is the inorganic compound having the formula KN3. It is a white, water-soluble salt. It is used as a reagent in the laboratory.
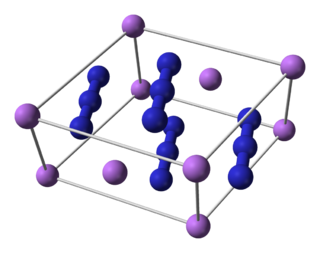
Lithium azide is the lithium salt of hydrazoic acid. It is an unstable and toxic compound that decomposes into lithium and nitrogen when heated.

Nitrosyl azide is an inorganic compound of nitrogen and oxygen with the chemical formula N3−N=O. It is a highly labile nitrogen oxide with the empirical formula N4O.

Chlorine azide is an inorganic compound that was discovered in 1908 by Friedrich Raschig. Concentrated ClN3 is notoriously unstable and may spontaneously detonate at any temperature.
Silicon tetraazide is a thermally unstable binary compound of silicon and nitrogen with a nitrogen content of 85.7%. This high-energy compound combusts spontaneously and can only be studied in a solution. A further coordination to a six-fold coordinated structure such as a hexaazidosilicate ion [Si(N3)6]2− or as an adduct with bicationic ligands Si(N3)4·L2 will result in relatively stable, crystalline solids that can be handled at room temperature.

4-Chlorophenyl azide is an organic aryl azide compound with the chemical formula C6H4ClN3. The geometry between the nitrogen atoms in the azide functional group is approximately linear while the geometry between the nitrogen and the carbon of the benzene is trigonal planar.

Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3. Its properties resemble those of ClN3, BrN3, and IN3. The bond between the fluorine atom and the nitrogen is very weak, leading to this substance being very unstable and prone to explosion. Calculations show the F–N–N angle to be around 102° with a straight line of 3 nitrogen atoms.

Rubidium azide is an inorganic compound with the formula RbN3. It is the rubidium salt of the hydrazoic acid HN3. Like most azides, it is explosive.

Caesium azide or cesium azide is an inorganic compound of caesium and nitrogen. It is a salt of azide with the formula CsN3.

Boron triazide, also known as triazidoborane, is a thermally unstable compound of boron and nitrogen with a nitrogen content of 92.1 %. Formally, it is the triazido derivative of borane and is a covalent inorganic azide. The high-energy compound, which has the propensity to undergo spontaneous explosive decomposition, was first described in 1954 by Egon Wiberg and Horst Michaud of the University of Munich.

Sulfuryl diazide or sulfuryl azide is a chemical compound with the molecular formula SO2(N3)2. It was first described in the 1920s when its reactions with benzene and p-xylene were studied by Theodor Curtius and Karl Friedrich Schmidt. The compound is reported as having "exceedingly explosive, unpredictable properties" and "in many cases very violent explosions occurred without any apparent reason".
An organic azide is an organic compound that contains an azide functional group. Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Low molecular weight azides are considered especially hazardous and are avoided. In the research laboratory, azides are precursors to amines. They are also popular for their participation in the "click reaction" between an azide and an alkyne and in Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry.

Transition metal azide complexes are coordination complexes containing one or more azide (N3−) ligands.
References
- ↑ Frierson, W. Joe; Kronrad, J.; Browne, A. W. (September 1943). "Chlorine Azide, ClN3 I". Journal of the American Chemical Society. 65 (9): 1696–1698. doi:10.1021/ja01249a012.