Related Research Articles

Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.
In chemistry, azide is a linear, polyatomic anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.

Sodium azide is an inorganic compound with the formula NaN3. This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance, is highly soluble in water, and is very acutely poisonous.

Silver azide is the chemical compound with the formula AgN3. It is a silver(I) salt of hydrazoic acid. It forms a colorless crystals. Like most azides, it is a primary explosive.

Thallium azide, TlN3, is a yellow-brown crystalline solid poorly soluble in water. Although it is not nearly as sensitive to shock or friction as lead azide, it can easily be detonated by a flame or spark. It can be stored safely dry in a closed non-metallic container.

Copper(II) azide is a medium density explosive with the molecular formula Cu(N3)2.
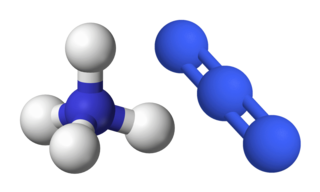
Ammonium azide is the chemical compound with the formula [NH4]N3, being the salt of ammonia and hydrazoic acid. Like other inorganic azides, this colourless crystalline salt is a powerful explosive, although it has a remarkably low sensitivity. [NH4]N3 is physiologically active and inhalation of small amounts causes headaches and palpitations. It was first obtained by Theodor Curtius in 1890, along with other azides.

Potassium azide is the inorganic compound having the formula KN3. It is a white, water-soluble salt. It is used as a reagent in the laboratory.
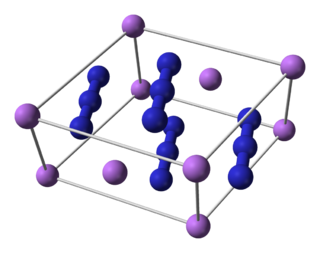
Lithium azide is the lithium salt of hydrazoic acid. It is an unstable and toxic compound that decomposes into lithium and nitrogen when heated.
Barium azide is an inorganic azide with the formula Ba(N3)2. It is a barium salt of hydrazoic acid. Like most azides, it is explosive. It is less sensitive to mechanical shock than lead azide.

Chlorine azide is an inorganic compound that was discovered in 1908 by Friedrich Raschig. Concentrated ClN3 is notoriously unstable and may spontaneously detonate at any temperature.

Bromine azide is an explosive inorganic compound with the formula BrN3. It has been described as a crystal or a red liquid at room temperature. It is extremely sensitive to small variations in temperature and pressure, with explosions occurring at Δp ≥ 0.05 Torr and also upon crystallization, thus extreme caution must be observed when working with this chemical.

Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3. Its properties resemble those of ClN3, BrN3, and IN3. The bond between the fluorine atom and the nitrogen is very weak, leading to this substance being very unstable and prone to explosion. Calculations show the F–N–N angle to be around 102° with a straight line of 3 nitrogen atoms.
Tellurium nitride describes chemical compounds of Te containing N3−. Efforts have been made toward the binary nitrides but the results are inconclusive and it appears that such materials are unstable. Still unconfirmed is Te4N4, which would be an analogue of tetraselenium tetranitride (Se4N4) and tetrasulfur tetranitride (S4N4). It has long been known that ammonia reacts with tellurium tetrachloride, which is similar to the method of synthesis of S4N4. The reaction of TeCl4 with a THF solution of N(SiMe3)3 gives a well-defined tellurium nitride [Te6N8(TeCl2)4(THF)4].

Rubidium azide is an inorganic compound with the formula RbN3. It is the rubidium salt of the hydrazoic acid HN3. Like most azides, it is explosive.
Zinc azideZn(N3)2 is an inorganic compound composed of zinc cations (Zn2+) and azide anions (N−3). It is a white, explosive solid that can be prepared by the protonolysis of diethylzinc with hydrazoic acid:

Caesium azide or cesium azide is an inorganic compound of caesium and nitrogen. It is a salt of azide with the formula CsN3.

Transition metal azide complexes are coordination complexes containing one or more azide (N3−) ligands.
Homoleptic azido compounds are chemical compounds in which the only anion or ligand is the azide group, -N3. The breadth of homoleptic azide compounds spans nearly the entire periodic table. With rare exceptions azido compounds are highly shock sensitive and need to be handled with the upmost caution. Binary azide compounds can take on several different structures including discrete compounds, or one- two, and three-dimensional nets, leading some to dub them as "polyazides". Reactivity studies of azide compounds are relatively limited due to how sensitive they can be. The sensitivity of these compounds tends to be correlated with the amount of ionic or covalent character the azide-element bond has, with ionic character being far more stable than covalent character. Therefore, compounds such as silver or sodium azide – which have strong ionic character – tend to possess more synthetic utility than their covalent counterparts. A few other notable exceptions include polymeric networks which possess unique magnetic properties, group 13 azides which unlike most other azides decompose to nitride compounds (important materials for semiconductors), other limited uses as synthetic reagents for the transfer for azide groups, or interest in high energy density materials.
References
- ↑ Klapötke, Thomas M.; Krumm, Burkhard; Mayer, Peter; Schwab, Ingo (8 December 2003). "Binary Tellurium(IV) Azides: Te(N3)4 and [Te(N3)5]−". Angewandte Chemie. 42 (47): 5843–5846. doi:10.1002/anie.200352656 . Retrieved 3 November 2023.